NPs Basic Information

|
Name |
1,2,3,4-Tetramethylbenzene
|
| Molecular Formula | C10H14 | |
| IUPAC Name* |
1,2,3,4-tetramethylbenzene
|
|
| SMILES |
CC1=C(C(=C(C=C1)C)C)C
|
|
| InChI |
InChI=1S/C10H14/c1-7-5-6-8(2)10(4)9(7)3/h5-6H,1-4H3
|
|
| InChIKey |
UOHMMEJUHBCKEE-UHFFFAOYSA-N
|
|
| Synonyms |
1,2,3,4-TETRAMETHYLBENZENE; 488-23-3; Prehnitene; Prehnitol; Benzene, 1,2,3,4-tetramethyl-; TETRAMETHYLBENZENE; 96WT7D2WXJ; 1,2,3,4-tetramethyl-benzene; 25619-60-7; CHEBI:38997; MFCD00008521; NSC-93932; Benzene, tetramethyl-; EINECS 207-673-1; NSC 93932; UNII-96WT7D2WXJ; Prehenitene; CCRIS 8659; Tetramethylbenzenes; tetra-methyl benzene; 1,3,4-Tetramethylbenzene; UNII-5L4Y5PFP1R; 5L4Y5PFP1R; Benzene,2,3,4-tetramethyl-; CHEMBL1797278; DTXSID4060072; Tetramethylbenzene (all isomers); AMY25690; BCP07046; NSC93932; ZINC1609579; 1,2,3,4-TETRAMETHLYBENZENE; 1,2,3,4-TRITRAMETHYLBENZENE; AKOS009031131; CS-W006416; DS-7452; HY-W006416; s10933; SY261146; DB-018721; FT-0606203; 488T233; A871875; Q27118087; 1,2,3,4-Tetramethylbenzene 100 microg/mL in Methanol
|
|
| CAS | 488-23-3 | |
| PubChem CID | 10263 | |
| ChEMBL ID | CHEMBL1797278 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 134.22 | ALogp: | 4.0 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 1 |
| Heavy Atoms: | 10 | QED Weighted: | 0.507 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.432 | MDCK Permeability: | 0.00002550 |
| Pgp-inhibitor: | 0.002 | Pgp-substrate: | 0.142 |
| Human Intestinal Absorption (HIA): | 0.006 | 20% Bioavailability (F20%): | 0.944 |
| 30% Bioavailability (F30%): | 0.975 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.834 | Plasma Protein Binding (PPB): | 91.86% |
| Volume Distribution (VD): | 1.302 | Fu: | 8.15% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.885 | CYP1A2-substrate: | 0.943 |
| CYP2C19-inhibitor: | 0.563 | CYP2C19-substrate: | 0.918 |
| CYP2C9-inhibitor: | 0.313 | CYP2C9-substrate: | 0.81 |
| CYP2D6-inhibitor: | 0.499 | CYP2D6-substrate: | 0.934 |
| CYP3A4-inhibitor: | 0.232 | CYP3A4-substrate: | 0.509 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 11.758 | Half-life (T1/2): | 0.372 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.012 | Human Hepatotoxicity (H-HT): | 0.07 |
| Drug-inuced Liver Injury (DILI): | 0.165 | AMES Toxicity: | 0.23 |
| Rat Oral Acute Toxicity: | 0.02 | Maximum Recommended Daily Dose: | 0.459 |
| Skin Sensitization: | 0.339 | Carcinogencity: | 0.721 |
| Eye Corrosion: | 0.987 | Eye Irritation: | 0.997 |
| Respiratory Toxicity: | 0.024 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000364 |  |
0.563 | D0U3DU |  |
0.308 | ||
| ENC000180 |  |
0.471 | D0X0RI |  |
0.298 | ||
| ENC000181 |  |
0.444 | D01PJR |  |
0.286 | ||
| ENC000614 |  |
0.389 | D0X4RN |  |
0.263 | ||
| ENC000907 |  |
0.378 | D05VIX |  |
0.254 | ||
| ENC000098 |  |
0.367 | D06GIP |  |
0.250 | ||
| ENC000498 |  |
0.359 | D05FTJ | 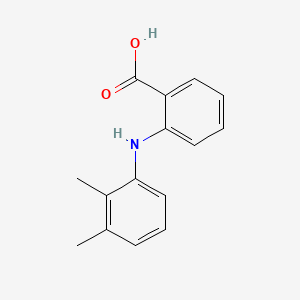 |
0.246 | ||
| ENC002097 |  |
0.353 | D0FA2O |  |
0.237 | ||
| ENC000728 |  |
0.350 | D0G7DJ |  |
0.231 | ||
| ENC005230 |  |
0.350 | D0Y4DY |  |
0.230 | ||