NPs Basic Information
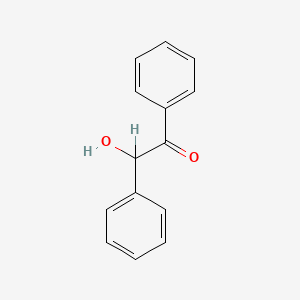
|
Name |
Benzoin
|
| Molecular Formula | C14H12O2 | |
| IUPAC Name* |
2-hydroxy-1,2-diphenylethanone
|
|
| SMILES |
C1=CC=C(C=C1)C(C(=O)C2=CC=CC=C2)O
|
|
| InChI |
InChI=1S/C14H12O2/c15-13(11-7-3-1-4-8-11)14(16)12-9-5-2-6-10-12/h1-10,13,15H
|
|
| InChIKey |
ISAOCJYIOMOJEB-UHFFFAOYSA-N
|
|
| Synonyms |
BENZOIN; 119-53-9; 2-Hydroxy-1,2-diphenylethanone; 2-Hydroxy-2-phenylacetophenone; Benzoylphenylcarbinol; DL-BENZOIN; Ethanone, 2-hydroxy-1,2-diphenyl-; 579-44-2; alpha-Hydroxybenzyl phenyl ketone; Phenylbenzoyl carbinol; (+-)-Benzoin; 2-hydroxy-1,2-diphenylethan-1-one; alpha-Hydroxy-alpha-phenylacetophenone; Aerozoin; Phenyl-alpha-hydroxybenzyl ketone; FEMA No. 2132; NCI-C50011; Acetophenone, 2-hydroxy-2-phenyl-; NSC 8082; Wy 42956; Hydroxy-2-phenyl acetophenone; 2-hydroxy-1,2-diphenyl-ethanone; 2-hydroxy-1,2-di(phenyl)ethanone; NSC-8082; a-Hydroxybenzyl phenyl ketone; CHEMBL190677; L7J6A1NE81; CHEBI:17682; 2-hydroxy-1,2-diphenyl ethanone; Phenyl-.alpha.-hydroxybenzyl ketone; Ketone, .alpha.-hydroxybenzyl phenyl; NCGC00091396-02; .alpha.-Hydroxy-.alpha.-phenylacetophenone; DSSTox_CID_144; DSSTox_RID_75397; DSSTox_GSID_20144; desyl alcohol; DL-Benzoin; Desyl alcohol;(+/-)-2-Hydroxy-2-phenylacetophenone; CCRIS 75; CAS-119-53-9; (RS)-Benzoin; Benzoin (VAN); HSDB 384; Ketone, alpha-hydroxybenzyl phenyl; EINECS 204-331-3; EINECS 209-441-5; MFCD00004496; Fenyl-alpha-hydroxybenzylketon [Czech]; benzoine; BRN 0391839; UNII-L7J6A1NE81; Fenyl-alpha-hydroxybenzylketon; WY-42956; AI3-00851; Benzoin absolute; CCRIS 9123; Alpha-hydroxy-a-phenylacetophenone; PhCH(OH)COPh; PhCOCH(OH)Ph; Benzoin, 98%; (+/-)-benzoin; (1)-2-Hydroxy-1,2-diphenylethan-1-one; Benzoin, >=98%; BENZOIN [MI]; (.+/-.)-Benzoin; WLN: QYR&VR; SCHEMBL145; EC 204-331-3; Hyperabsolute benzoin, Siam; Benzoin, analytical standard; Oprea1_687165; 4-08-00-01279 (Beilstein Handbook Reference); 9000-72-0; MLS002152893; a-Hydroxy-a-phenylacetophenone; FEMA No. 2133; 2-hydroxy-2-phenyl-acetophenone; BENZOIN, (+/-)-; DTXSID1020144; Fenyl-.alpha.-hydroxybenzylketon; BDBM22728; FEMA 2132; HSDB 1929; NSC8082; alpha -Hydroxybenzyl phenyl ketone; HMS3039I03; Phenyl-alpha -hydroxybenzyl ketone; .alpha.-Hydroxybenzyl phenyl ketone; HY-B1550; Tox21_111126; Tox21_201888; Tox21_302790; STK358785; AKOS000118894; AKOS016038141; Tox21_111126_1; 2-Hydroxy-1,2-diphenylethanone, 9CI; Benzoin 100 microg/mL in Acetonitrile; CS-W020562; DB14020; alpha -Hydroxy-alpha -phenylacetophenone; NCGC00091396-01; NCGC00091396-03; NCGC00091396-05; NCGC00256433-01; NCGC00259437-01; AC-11139; Benzoin, Vetec(TM) reagent grade, 98%; BS-14748; SMR001224505; DB-018065; B0079; B0222; Benzoin Zone Refined (number of passes:40); Benzoin, purified by sublimation, >=99.5%; FT-0612530; FT-0626841; FT-0635908; FT-0635909; EN300-18095; C01408; D77908; (+/-)-2-HYDROXY-1,2-DIPHENYLETHANONE; A804309; AE-848/06163047; Ethanone, 2-hydroxy-1,2-diphenyl-, (.+/-.)-; Q426819; SR-01000854680; J-004149; J-509605; SR-01000854680-2; Z57160197; F0001-0307; Ethanone, 2-hydroxy-1,2-diphenyl-, mixt. with aloe, storax and Tolu Balsam, tincture
|
|
| CAS | 119-53-9 | |
| PubChem CID | 8400 | |
| ChEMBL ID | CHEMBL190677 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 212.24 | ALogp: | 2.1 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 3 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 37.3 | Aromatic Rings: | 2 |
| Heavy Atoms: | 16 | QED Weighted: | 0.792 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.667 | MDCK Permeability: | 0.00002650 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.004 |
| Human Intestinal Absorption (HIA): | 0.028 | 20% Bioavailability (F20%): | 0.792 |
| 30% Bioavailability (F30%): | 0.017 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.509 | Plasma Protein Binding (PPB): | 90.97% |
| Volume Distribution (VD): | 0.548 | Fu: | 3.84% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.474 | CYP1A2-substrate: | 0.111 |
| CYP2C19-inhibitor: | 0.498 | CYP2C19-substrate: | 0.21 |
| CYP2C9-inhibitor: | 0.341 | CYP2C9-substrate: | 0.18 |
| CYP2D6-inhibitor: | 0.014 | CYP2D6-substrate: | 0.305 |
| CYP3A4-inhibitor: | 0.022 | CYP3A4-substrate: | 0.315 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.24 | Half-life (T1/2): | 0.564 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.028 | Human Hepatotoxicity (H-HT): | 0.034 |
| Drug-inuced Liver Injury (DILI): | 0.73 | AMES Toxicity: | 0.031 |
| Rat Oral Acute Toxicity: | 0.028 | Maximum Recommended Daily Dose: | 0.027 |
| Skin Sensitization: | 0.24 | Carcinogencity: | 0.048 |
| Eye Corrosion: | 0.003 | Eye Irritation: | 0.664 |
| Respiratory Toxicity: | 0.055 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000093 | 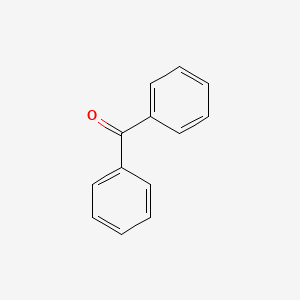 |
0.642 | D0G1VX | 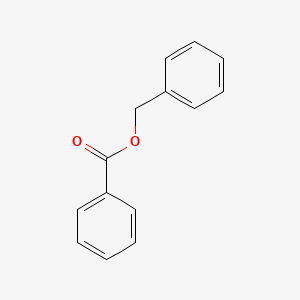 |
0.576 | ||
| ENC000077 | 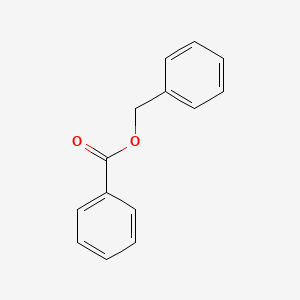 |
0.576 | D07HQC | 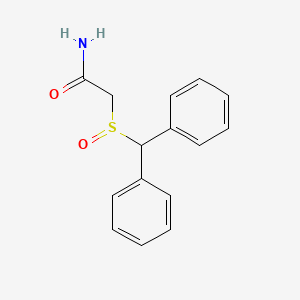 |
0.538 | ||
| ENC001449 |  |
0.554 | D0J5RN | 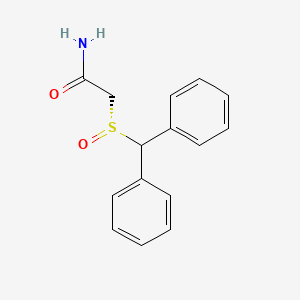 |
0.538 | ||
| ENC000461 | 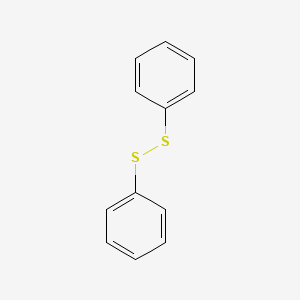 |
0.467 | D04DXN | 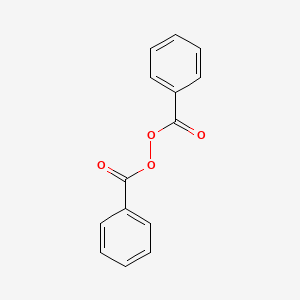 |
0.531 | ||
| ENC001523 | 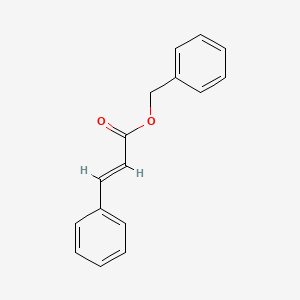 |
0.456 | D0W9WF | 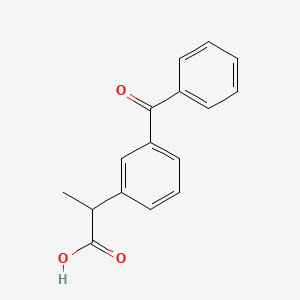 |
0.478 | ||
| ENC000326 | 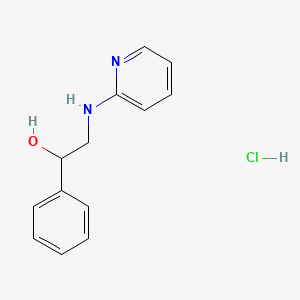 |
0.446 | D01FGR | 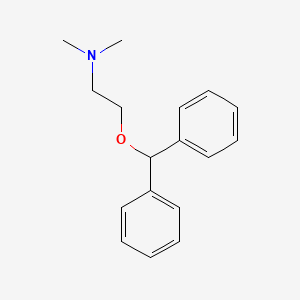 |
0.464 | ||
| ENC001428 | 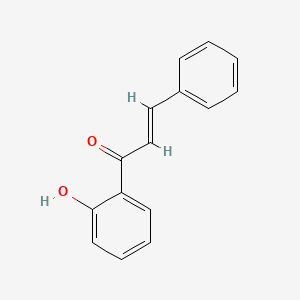 |
0.439 | D0W2AN | 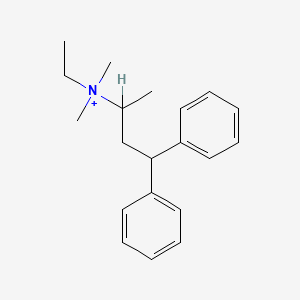 |
0.438 | ||
| ENC001737 | 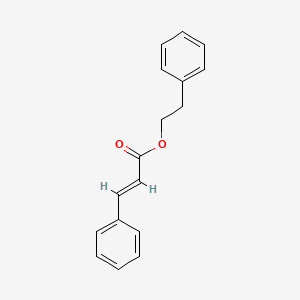 |
0.437 | D08HRJ | 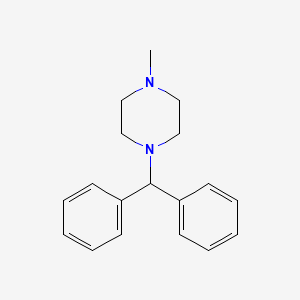 |
0.438 | ||
| ENC000651 | 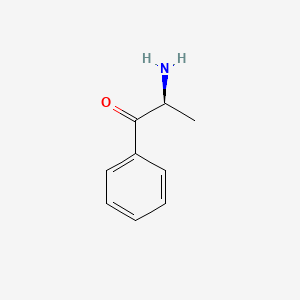 |
0.434 | D03XYW | 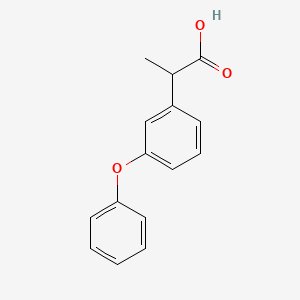 |
0.426 | ||
| ENC000013 | 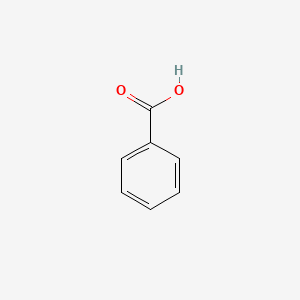 |
0.420 | D0E3OF |  |
0.423 | ||