NPs Basic Information
|
Name |
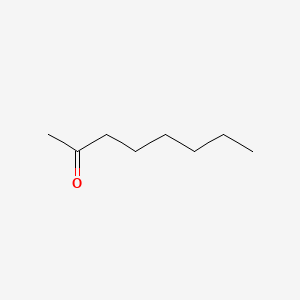
2-Octanone
|
| Molecular Formula | C8H16O | |
| IUPAC Name* |
octan-2-one
|
|
| SMILES |
CCCCCCC(=O)C
|
|
| InChI |
InChI=1S/C8H16O/c1-3-4-5-6-7-8(2)9/h3-7H2,1-2H3
|
|
| InChIKey |
ZPVFWPFBNIEHGJ-UHFFFAOYSA-N
|
|
| Synonyms |
2-OCTANONE; Octan-2-one; 111-13-7; Hexyl methyl ketone; n-Hexyl methyl ketone; Methyl hexyl ketone; Methyl n-hexyl ketone; 2-Oxooctane; Octanone; FEMA No. 2802; CHEMBL18549; J2G84H29AF; CHEBI:87434; NSC-3712; 2-Octanone (natural); FEMA Number 2802; HSDB 5545; NSC 3712; EINECS 203-837-1; BRN 0635843; UNII-J2G84H29AF; AI3-05617; octanone-2; octane-2-one; 2- octanone; Metyhl Hexyl Ketone; n-C6H13COCH3; 2-OCTANONE [FHFI]; 2-OCTANONE [HSDB]; SCHEMBL43776; 4-01-00-03339 (Beilstein Handbook Reference); 2-Octanone, analytical standard; 2-Octanone, >=98%, FG; DTXSID4021927; FEMA 2802; HEXYL METHYL KETONE [MI]; NSC3712; NSC5936; METHYL HEXYL KETONE [FCC]; 2-Octanone, natural, 98%, FG; 2-Octanone, reagent grade, 98%; NSC-5936; ZINC1672808; BBL011429; BDBM50028815; LMFA12000054; MFCD00009540; STL146536; AKOS005720775; CS-W011125; DA-16705; VS-02947; FT-0613243; O0038; EN300-20060; E76016; A802298; J-002527; Q18611679
|
|
| CAS | 111-13-7 | |
| PubChem CID | 8093 | |
| ChEMBL ID | CHEMBL18549 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 128.21 | ALogp: | 2.4 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 5 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 17.1 | Aromatic Rings: | 0 |
| Heavy Atoms: | 9 | QED Weighted: | 0.519 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.365 | MDCK Permeability: | 0.00002210 |
| Pgp-inhibitor: | 0.011 | Pgp-substrate: | 0.004 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.933 |
| 30% Bioavailability (F30%): | 0.966 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.998 | Plasma Protein Binding (PPB): | 81.83% |
| Volume Distribution (VD): | 0.695 | Fu: | 25.93% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.861 | CYP1A2-substrate: | 0.899 |
| CYP2C19-inhibitor: | 0.35 | CYP2C19-substrate: | 0.797 |
| CYP2C9-inhibitor: | 0.186 | CYP2C9-substrate: | 0.91 |
| CYP2D6-inhibitor: | 0.014 | CYP2D6-substrate: | 0.576 |
| CYP3A4-inhibitor: | 0.024 | CYP3A4-substrate: | 0.153 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.481 | Half-life (T1/2): | 0.789 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.053 | Human Hepatotoxicity (H-HT): | 0.029 |
| Drug-inuced Liver Injury (DILI): | 0.095 | AMES Toxicity: | 0.006 |
| Rat Oral Acute Toxicity: | 0.042 | Maximum Recommended Daily Dose: | 0.034 |
| Skin Sensitization: | 0.44 | Carcinogencity: | 0.114 |
| Eye Corrosion: | 0.986 | Eye Irritation: | 0.978 |
| Respiratory Toxicity: | 0.076 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000454 | 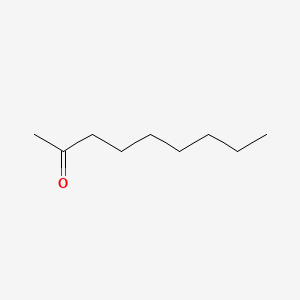 |
0.889 | D0FD0H |  |
0.412 | ||
| ENC000250 |  |
0.875 | D0AY9Q |  |
0.375 | ||
| ENC000451 | 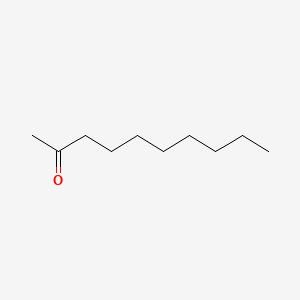 |
0.800 | D01QLH |  |
0.353 | ||
| ENC000265 |  |
0.727 | D0UU9Y | 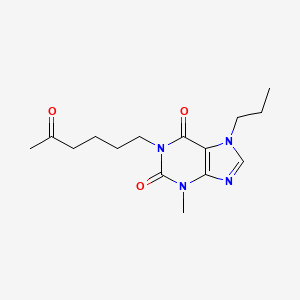 |
0.313 | ||
| ENC000556 | 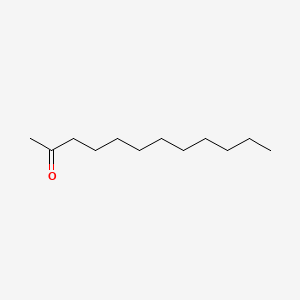 |
0.667 | D0E4WR |  |
0.311 | ||
| ENC000399 | 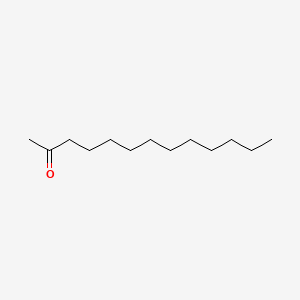 |
0.615 | D0Z5BC |  |
0.304 | ||
| ENC001025 |  |
0.600 | D03ZJE | 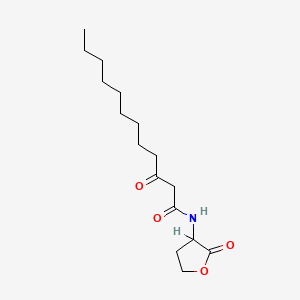 |
0.297 | ||
| ENC000030 | 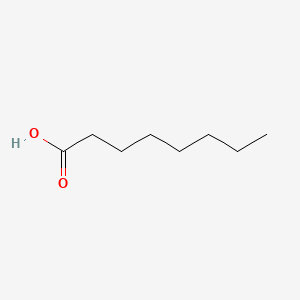 |
0.594 | D0XN8C |  |
0.297 | ||
| ENC000687 | 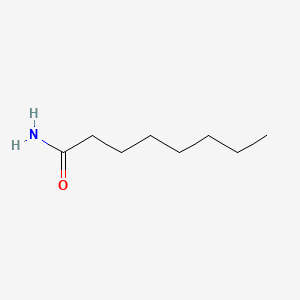 |
0.594 | D07ILQ |  |
0.292 | ||
| ENC000253 | 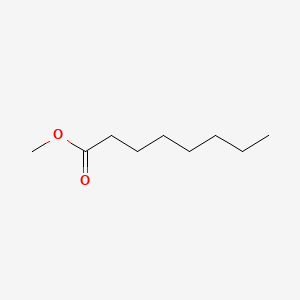 |
0.588 | D0UE9X |  |
0.286 | ||