NPs Basic Information

|
Name |
gamma-Valerolactone
|
| Molecular Formula | C5H8O2 | |
| IUPAC Name* |
5-methyloxolan-2-one
|
|
| SMILES |
CC1CCC(=O)O1
|
|
| InChI |
InChI=1S/C5H8O2/c1-4-2-3-5(6)7-4/h4H,2-3H2,1H3
|
|
| InChIKey |
GAEKPEKOJKCEMS-UHFFFAOYSA-N
|
|
| Synonyms |
gamma-Valerolactone; 108-29-2; 4-Pentanolide; 4-Valerolactone; 5-methyldihydrofuran-2(3H)-one; 5-methyloxolan-2-one; 4-Hydroxypentanoic acid lactone; gamma-Pentalactone; 4-Hydroxyvaleric acid lactone; 2(3H)-Furanone, dihydro-5-methyl-; 4-Methyl-gamma-butyrolactone; gamma-Methyl-gamma-butyrolactone; 4-Methyl-4-hydroxybutanoic acid lactone; DIHYDRO-5-METHYL-2(3H)-FURANONE; .gamma.-Valerolactone; 5-Methyltetrahydro-2-furanone; NSC 33700; FEMA No. 3103; 2(3H)-Furanone, dihydr-5-methyl-; .gamma.-Methyl-.gamma.-butyrolactone; gamma-Pentanolactone; .gamma.-Pentalactone; Valeric acid, 4-hydroxy-, gamma-lactone; MFCD00005400; CHEMBL195593; 4-Methyl-.gamma.-butyrolactone; CHEBI:48569; 5-Methyldihydro-2(3H)-furanone; Dihydro-5-methylfuran-2(3H)-one; O7056XK37X; NSC-33700; Valeric acid, 4-hydroxy-, .gamma.-lactone; Pentanoic acid, 4-hydroxy-, .gamma.-lactone; Pentanolide-1,4; 219630-19-0; gamma-Valeryllactone; gamma-Valerolakton; gamma-Valerolakton [Czech]; Gamma Valerolactone; CCRIS 3597; EINECS 203-569-5; BRN 0080420; 5-methyltetrahydrofuran-2-one; y-Valerolactone; Pentanoic acid, 4-hydroxy-, gamma-lactone; UNII-O7056XK37X; AI3-04327; gamma -Valerolactone; .gamma.-Valerolakton; .gamma.-Pentanolactone; 4-Methyl-g-butyrolactone; DSSTox_CID_27618; DSSTox_RID_82456; DSSTox_GSID_47618; SCHEMBL37255; Dihydro-5-methyl-2-furanone; 5-17-09-00024 (Beilstein Handbook Reference); 5-methyl-dihydro-furan-2-one; DTXSID0047618; Pentanoic acid, .gamma.-lactone; GAMMA-VALEROLACTONE [FCC]; (.+/-.)-4-Methylbutyrolactone; (.+/-.)-.gamma.-Valerolactone; (RS)-.GAMMA.-PENTALACTONE; NSC33700; (3-Amino-pyridin-2-yl)-aceticacid; 5-Methyldihydro-2(3H)-furanone #; dihydro-5-methyl-2(3H)-furanone,; Tox21_302624; BBL011475; BDBM50168010; LMFA07040008; STL146587; .GAMMA.-VALEROLACTONE [FHFI]; AKOS005206963; gamma-Valerolactone,delta-Valerolactone; CS-W016654; DS-4944; gamma-Valerolactone, analytical standard; gamma-Valerolactone, >=99%, FCC, FG; gamma-Valerolactone, natural, 95%, FG; NCGC00256671-01; BP-31066; CAS-108-29-2; SY011304; (+/-)-.GAMMA.-METHYLBUTYROLACTONE; DB-003681; gamma-Valerolactone, ReagentPlus(R), 99%; FT-0605160; FT-0626628; V0007; EN300-61318; 4-HYDROXYPENTANOIC ACID .GAMMA.-LACTONE; P20539; A895293; Q845530; 2(3H)-Furanone, dihydro-5-methyl-, (.+/-.)-; gamma-Valerolactone, Vetec(TM) reagent grade, 98%; J-002085; F0001-0163; Z963595138; 4,5-Dihydro-5-methyl-2(3H)-furanone~4-Hydroxypentanoic acid lactone~gamma-Pentanolactone
|
|
| CAS | 108-29-2 | |
| PubChem CID | 7921 | |
| ChEMBL ID | CHEMBL195593 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 100.12 | ALogp: | 0.6 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 26.3 | Aromatic Rings: | 1 |
| Heavy Atoms: | 7 | QED Weighted: | 0.426 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.362 | MDCK Permeability: | 0.00008490 |
| Pgp-inhibitor: | 0.012 | Pgp-substrate: | 0.001 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.002 |
| 30% Bioavailability (F30%): | 0.498 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.991 | Plasma Protein Binding (PPB): | 28.81% |
| Volume Distribution (VD): | 0.791 | Fu: | 68.85% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.441 | CYP1A2-substrate: | 0.118 |
| CYP2C19-inhibitor: | 0.044 | CYP2C19-substrate: | 0.612 |
| CYP2C9-inhibitor: | 0.011 | CYP2C9-substrate: | 0.505 |
| CYP2D6-inhibitor: | 0.009 | CYP2D6-substrate: | 0.394 |
| CYP3A4-inhibitor: | 0.021 | CYP3A4-substrate: | 0.257 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 10.232 | Half-life (T1/2): | 0.85 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.006 | Human Hepatotoxicity (H-HT): | 0.171 |
| Drug-inuced Liver Injury (DILI): | 0.397 | AMES Toxicity: | 0.08 |
| Rat Oral Acute Toxicity: | 0.023 | Maximum Recommended Daily Dose: | 0.26 |
| Skin Sensitization: | 0.722 | Carcinogencity: | 0.753 |
| Eye Corrosion: | 0.968 | Eye Irritation: | 0.961 |
| Respiratory Toxicity: | 0.048 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000456 |  |
0.708 | D0Z8AA |  |
0.348 | ||
| ENC005694 |  |
0.400 | D0Z8SF |  |
0.211 | ||
| ENC005373 |  |
0.375 | D0H1QY | 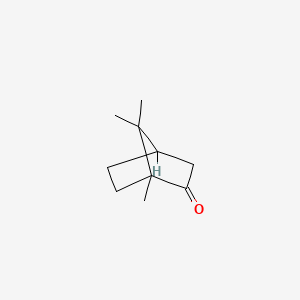 |
0.200 | ||
| ENC004080 | 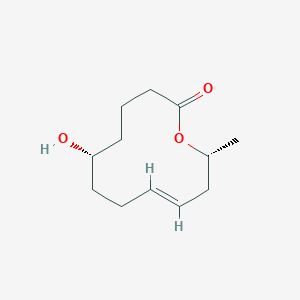 |
0.356 | D0K7LU | 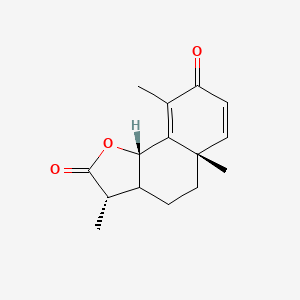 |
0.193 | ||
| ENC004081 |  |
0.356 | D0TY5N | 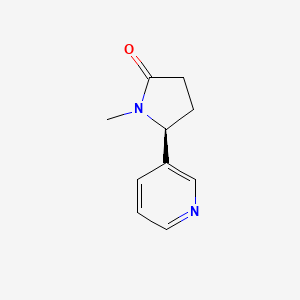 |
0.191 | ||
| ENC000525 | 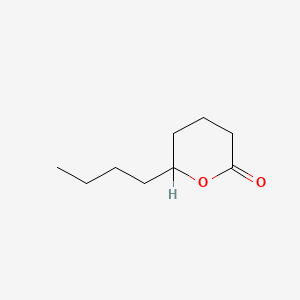 |
0.351 | D02WFK | 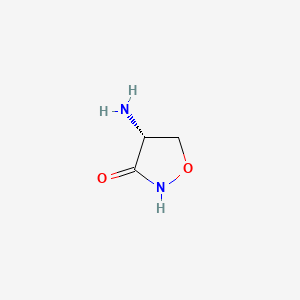 |
0.188 | ||
| ENC000899 |  |
0.341 | D0C7JF |  |
0.174 | ||
| ENC003475 | 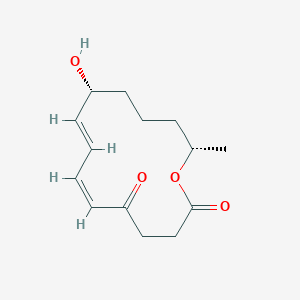 |
0.327 | D0S3WH |  |
0.172 | ||
| ENC001329 | 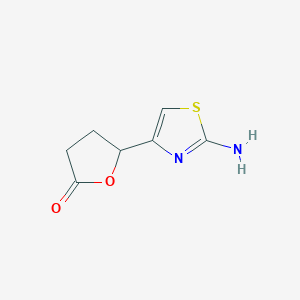 |
0.325 | D00EEL |  |
0.171 | ||
| ENC005378 |  |
0.321 | D0A2AJ |  |
0.169 | ||