NPs Basic Information

|
Name |
Acetate
|
| Molecular Formula | C2H3O2- | |
| IUPAC Name* |
acetate
|
|
| SMILES |
CC(=O)[O-]
|
|
| InChI |
InChI=1S/C2H4O2/c1-2(3)4/h1H3,(H,3,4)/p-1
|
|
| InChIKey |
QTBSBXVTEAMEQO-UHFFFAOYSA-M
|
|
| Synonyms |
acetate; Acetate Ion; Acetic acid, ion(1-); 71-50-1; Acetate ions; Acetate anion; Acetoxy ion; Acetic acid anion; MeCO2 anion; ethanoate; Acetate ion (1-); 569DQM74SC; Natriumazetat; Ethanoat; Shotgun; monoacetate; CHEMBL1354; UNII-569DQM74SC; Azetat; racemic acetate; acetyl hydroxide; Acetic acid ion; Acetic cid glacial; ACETATE [VANDF]; CH3-COO(-); DTXSID1037694; CHEBI:30089; BDBM50159793; CMC_13391; STL282721; AKOS022101130; DB14511; Q9154808
|
|
| CAS | 71-50-1 | |
| PubChem CID | 175 | |
| ChEMBL ID | CHEMBL1354 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 59.04 | ALogp: | 0.4 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 40.1 | Aromatic Rings: | 0 |
| Heavy Atoms: | 4 | QED Weighted: | 0.369 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.218 | MDCK Permeability: | 0.00216227 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.173 |
| Human Intestinal Absorption (HIA): | 0.008 | 20% Bioavailability (F20%): | 0.002 |
| 30% Bioavailability (F30%): | 0.002 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.923 | Plasma Protein Binding (PPB): | 11.63% |
| Volume Distribution (VD): | 0.323 | Fu: | 79.86% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.021 | CYP1A2-substrate: | 0.101 |
| CYP2C19-inhibitor: | 0.026 | CYP2C19-substrate: | 0.059 |
| CYP2C9-inhibitor: | 0.004 | CYP2C9-substrate: | 0.284 |
| CYP2D6-inhibitor: | 0.041 | CYP2D6-substrate: | 0.111 |
| CYP3A4-inhibitor: | 0.015 | CYP3A4-substrate: | 0.047 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 1.609 | Half-life (T1/2): | 0.791 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.01 | Human Hepatotoxicity (H-HT): | 0.15 |
| Drug-inuced Liver Injury (DILI): | 0.218 | AMES Toxicity: | 0.02 |
| Rat Oral Acute Toxicity: | 0.037 | Maximum Recommended Daily Dose: | 0.013 |
| Skin Sensitization: | 0.311 | Carcinogencity: | 0.033 |
| Eye Corrosion: | 0.973 | Eye Irritation: | 0.942 |
| Respiratory Toxicity: | 0.039 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000009 | 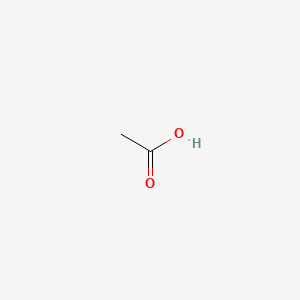 |
0.455 | D0Z4UY |  |
0.889 | ||
| ENC000288 | 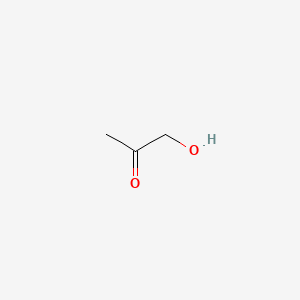 |
0.357 | D0C1PY |  |
0.889 | ||
| ENC000713 | 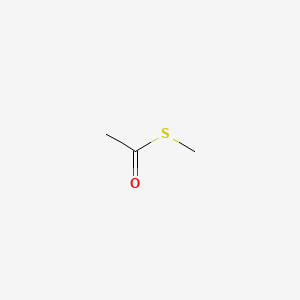 |
0.357 | D0Z4NI |  |
0.471 | ||
| ENC000061 | 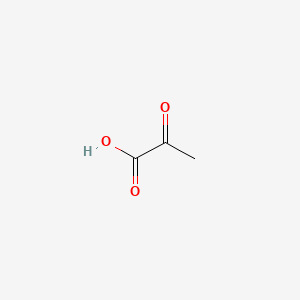 |
0.313 | D0F1GS |  |
0.471 | ||
| ENC000010 | 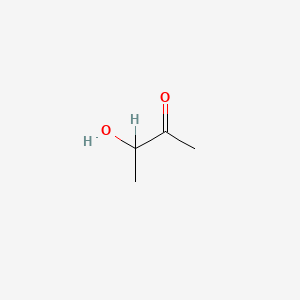 |
0.313 | D04CRL | 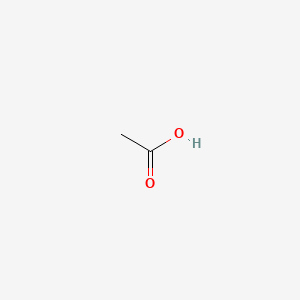 |
0.455 | ||
| ENC000015 |  |
0.286 | D02FLB |  |
0.417 | ||
| ENC000312 |  |
0.278 | D04UUH |  |
0.385 | ||
| ENC000058 |  |
0.267 | D00ZOF | 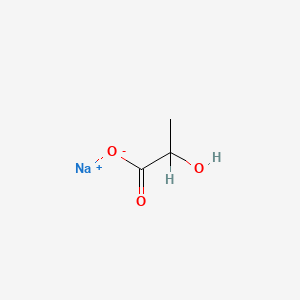 |
0.375 | ||
| ENC001900 | 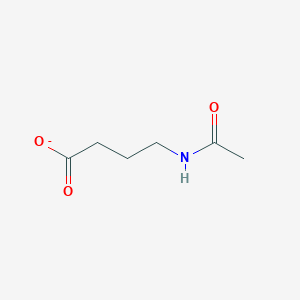 |
0.259 | D0R9BG | 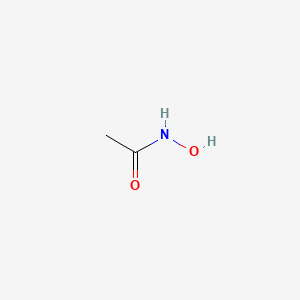 |
0.357 | ||
| ENC000403 | 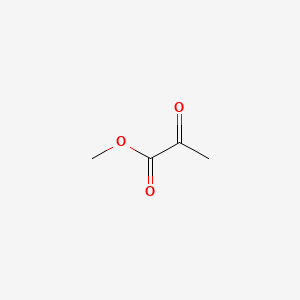 |
0.250 | D0G4JI | 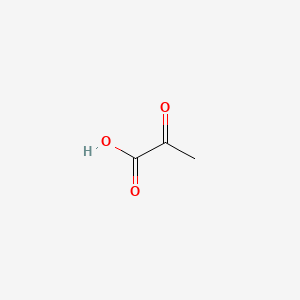 |
0.313 | ||