NPs Basic Information
|
Name |
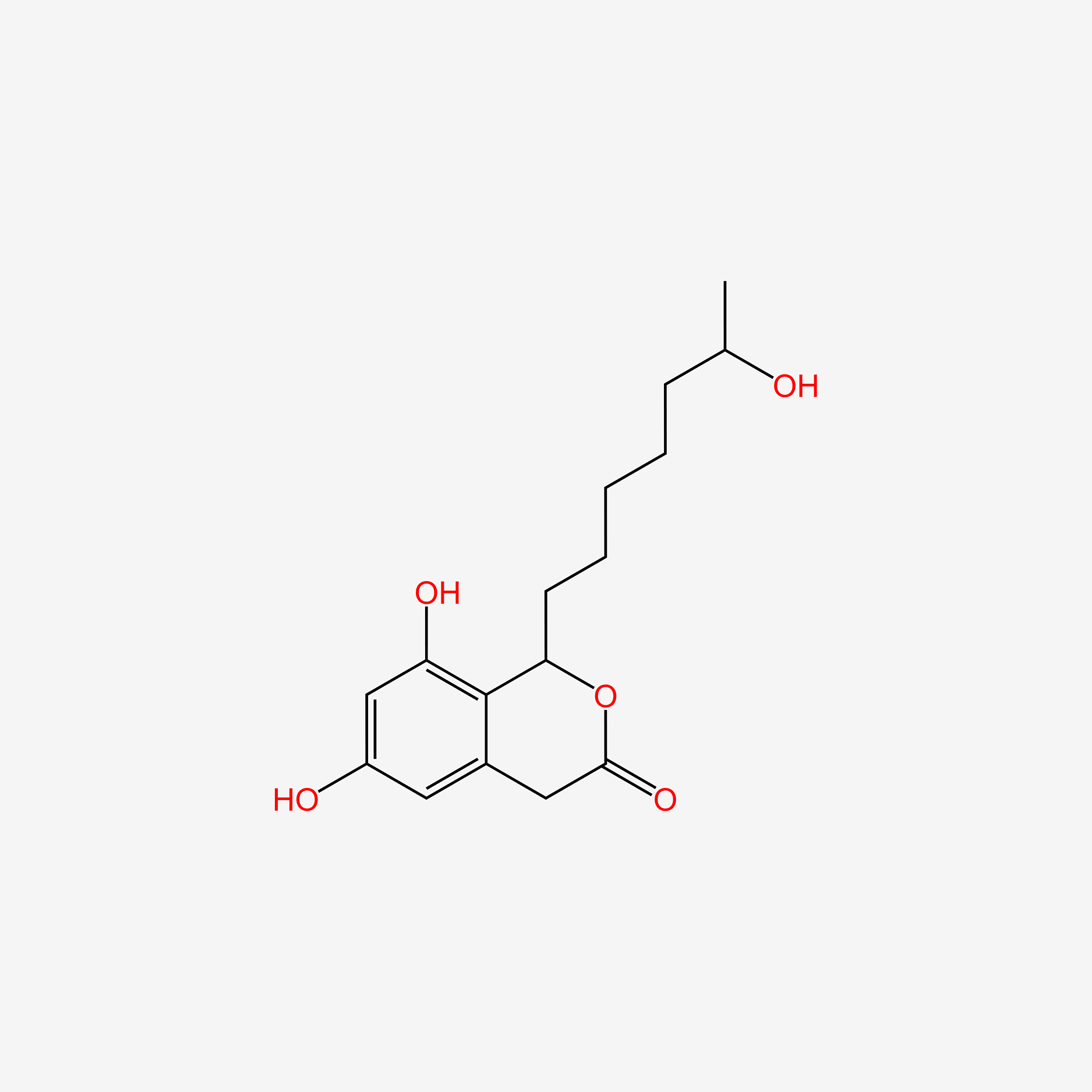
Dothiorelone H
|
| Molecular Formula | C16H22O5 | |
| IUPAC Name* |
6,8-dihydroxy-1-(6-hydroxyheptyl)-1,4-dihydroisochromen-3-one
|
|
| SMILES |
CC(O)CCCCCC1OC(=O)Cc2cc(O)cc(O)c21
|
|
| InChI |
InChI=1S/C16H22O5/c1-10(17)5-3-2-4-6-14-16-11(8-15(20)21-14)7-12(18)9-13(16)19/h7,9-10,14,17-19H,2-6,8H2,1H3
|
|
| InChIKey |
LWACOKGHBDKWPN-UHFFFAOYSA-N
|
|
| Synonyms |
NA
|
|
| CAS | NA | |
| PubChem CID | NA | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was calculated by STOUT. Reference: PMID:33906675.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 294.35 | ALogp: | 2.6 |
| HBD: | 3 | HBA: | 5 |
| Rotatable Bonds: | 6 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 87.0 | Aromatic Rings: | 2 |
| Heavy Atoms: | 21 | QED Weighted: | 0.553 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.767 | MDCK Permeability: | 0.00002520 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.742 |
| Human Intestinal Absorption (HIA): | 0.077 | 20% Bioavailability (F20%): | 0.969 |
| 30% Bioavailability (F30%): | 0.984 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.104 | Plasma Protein Binding (PPB): | 58.46% |
| Volume Distribution (VD): | 1.006 | Fu: | 31.92% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.254 | CYP1A2-substrate: | 0.571 |
| CYP2C19-inhibitor: | 0.51 | CYP2C19-substrate: | 0.32 |
| CYP2C9-inhibitor: | 0.587 | CYP2C9-substrate: | 0.975 |
| CYP2D6-inhibitor: | 0.083 | CYP2D6-substrate: | 0.404 |
| CYP3A4-inhibitor: | 0.415 | CYP3A4-substrate: | 0.25 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 14.196 | Half-life (T1/2): | 0.914 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.022 | Human Hepatotoxicity (H-HT): | 0.228 |
| Drug-inuced Liver Injury (DILI): | 0.3 | AMES Toxicity: | 0.183 |
| Rat Oral Acute Toxicity: | 0.257 | Maximum Recommended Daily Dose: | 0.873 |
| Skin Sensitization: | 0.718 | Carcinogencity: | 0.069 |
| Eye Corrosion: | 0.004 | Eye Irritation: | 0.187 |
| Respiratory Toxicity: | 0.257 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC002062 | 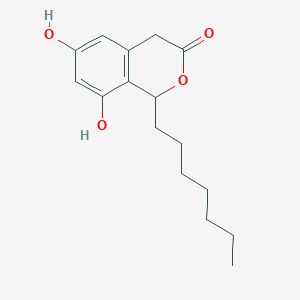 |
0.754 | D0L7AS | 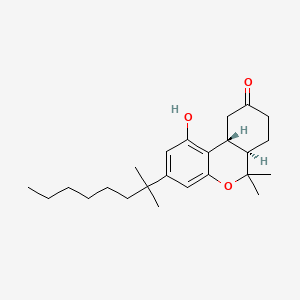 |
0.282 | ||
| ENC002053 | 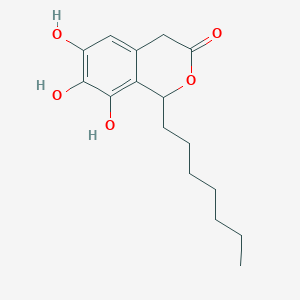 |
0.568 | D07MGA |  |
0.266 | ||
| ENC005793 |  |
0.506 | D0O1UZ | 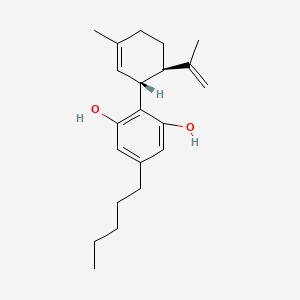 |
0.260 | ||
| ENC004666 |  |
0.444 | D0P1FO | 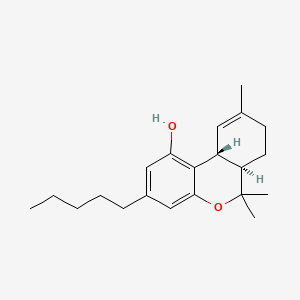 |
0.245 | ||
| ENC002006 | 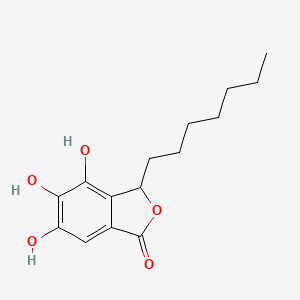 |
0.430 | D06KYN |  |
0.242 | ||
| ENC002685 |  |
0.429 | D02UFG | 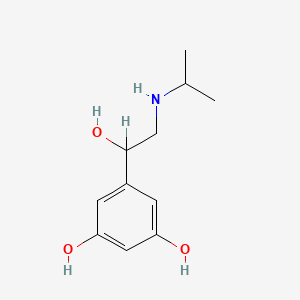 |
0.241 | ||
| ENC004669 |  |
0.424 | D0J7RK |  |
0.237 | ||
| ENC003741 |  |
0.424 | D0I4DQ |  |
0.233 | ||
| ENC004082 |  |
0.417 | D04XEG | 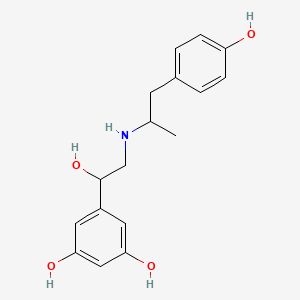 |
0.227 | ||
| ENC003189 |  |
0.414 | D01WUA |  |
0.223 | ||