NPs Basic Information
|
Name |
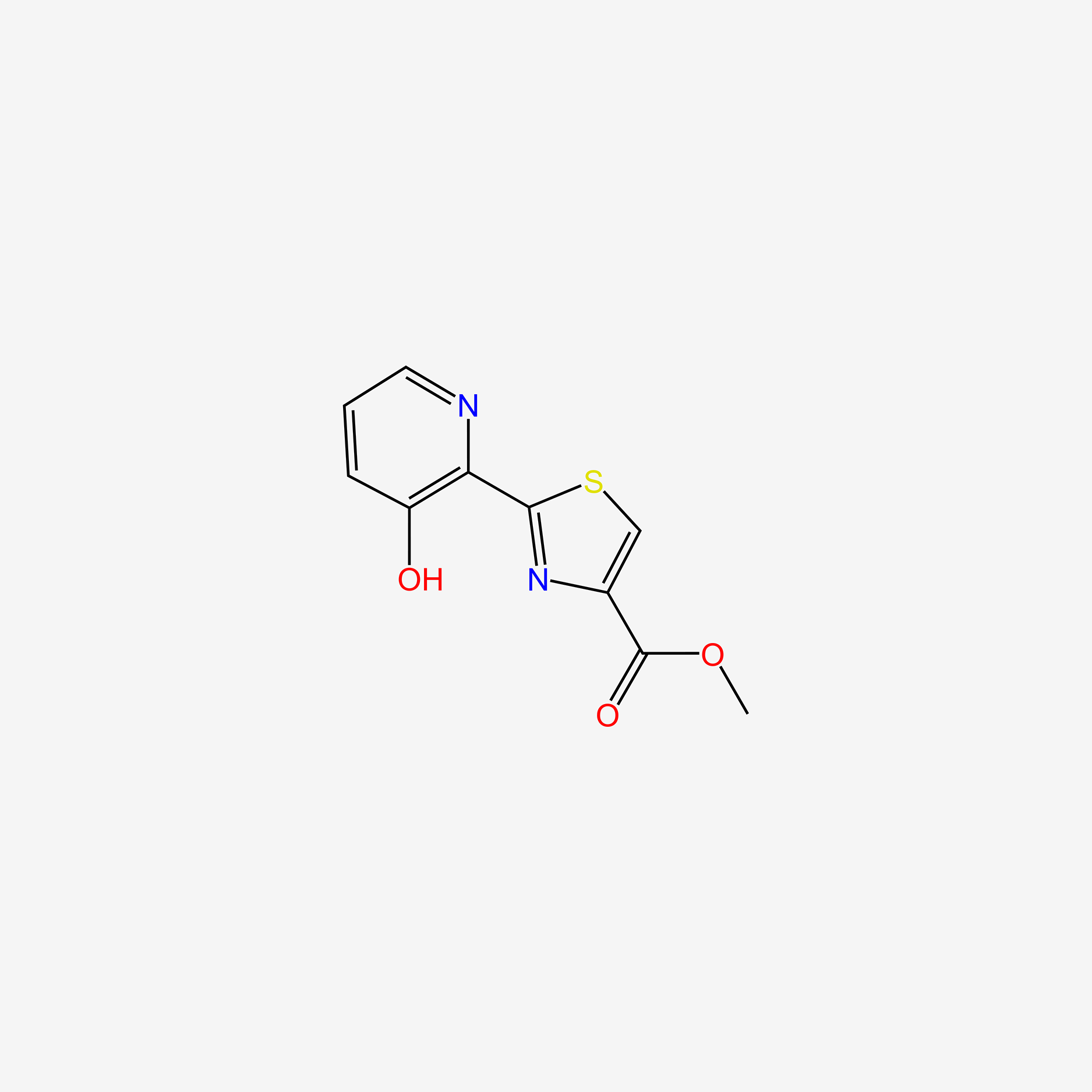
Methyl aeruginoate
|
| Molecular Formula | C10H8N2O3S | |
| IUPAC Name* |
methyl2-(3-hydroxypyridin-2-yl)-1,3-thiazole-4-carboxylate
|
|
| SMILES |
COC(=O)c1csc(-c2ncccc2O)n1
|
|
| InChI |
InChI=1S/C10H8N2O3S/c1-15-10(14)6-5-16-9(12-6)8-7(13)3-2-4-11-8/h2-5,13H,1H3
|
|
| InChIKey |
UIRNTEPNRZQPMX-UHFFFAOYSA-N
|
|
| Synonyms |
NA
|
|
| CAS | NA | |
| PubChem CID | NA | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was calculated by STOUT. Reference: PMID:33906675.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 236.25 | ALogp: | 1.7 |
| HBD: | 1 | HBA: | 6 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 72.3 | Aromatic Rings: | 2 |
| Heavy Atoms: | 16 | QED Weighted: | 0.81 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.877 | MDCK Permeability: | 0.00003620 |
| Pgp-inhibitor: | 0.002 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.019 | 20% Bioavailability (F20%): | 0.003 |
| 30% Bioavailability (F30%): | 0.474 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.555 | Plasma Protein Binding (PPB): | 88.54% |
| Volume Distribution (VD): | 0.459 | Fu: | 16.32% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.984 | CYP1A2-substrate: | 0.653 |
| CYP2C19-inhibitor: | 0.427 | CYP2C19-substrate: | 0.076 |
| CYP2C9-inhibitor: | 0.319 | CYP2C9-substrate: | 0.776 |
| CYP2D6-inhibitor: | 0.051 | CYP2D6-substrate: | 0.422 |
| CYP3A4-inhibitor: | 0.098 | CYP3A4-substrate: | 0.166 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 6.954 | Half-life (T1/2): | 0.651 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.015 | Human Hepatotoxicity (H-HT): | 0.215 |
| Drug-inuced Liver Injury (DILI): | 0.973 | AMES Toxicity: | 0.033 |
| Rat Oral Acute Toxicity: | 0.021 | Maximum Recommended Daily Dose: | 0.068 |
| Skin Sensitization: | 0.062 | Carcinogencity: | 0.039 |
| Eye Corrosion: | 0.004 | Eye Irritation: | 0.632 |
| Respiratory Toxicity: | 0.957 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC003520 | 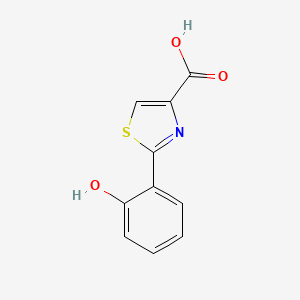 |
0.450 | D09SOA |  |
0.267 | ||
| ENC004704 |  |
0.413 | D06AEB |  |
0.266 | ||
| ENC000104 |  |
0.389 | D02HWP | 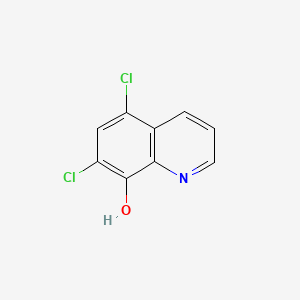 |
0.266 | ||
| ENC004705 |  |
0.333 | D07HBX | 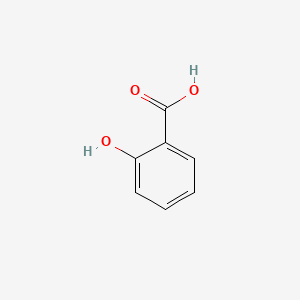 |
0.263 | ||
| ENC004415 |  |
0.333 | D0I6IB |  |
0.262 | ||
| ENC000195 |  |
0.316 | D0U0OT | 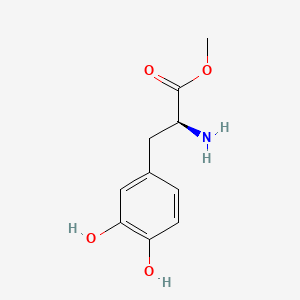 |
0.250 | ||
| ENC000056 | 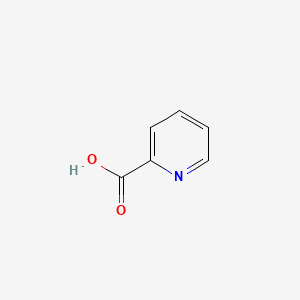 |
0.296 | D06NVJ | 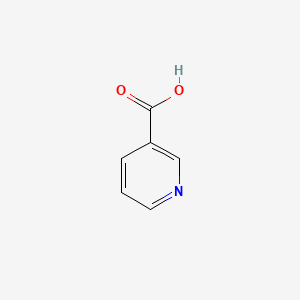 |
0.250 | ||
| ENC000193 |  |
0.295 | D0F5ZM | 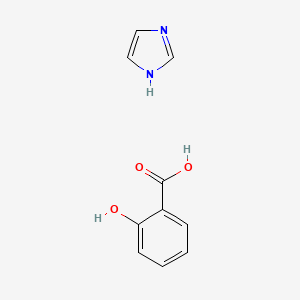 |
0.243 | ||
| ENC000729 | 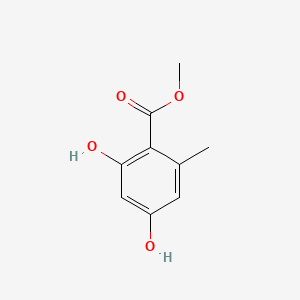 |
0.295 | D0Y0JH |  |
0.241 | ||
| ENC002283 |  |
0.295 | D0L5PO |  |
0.240 | ||