NPs Basic Information

|
Name |
alpha-Gurjunene
|
| Molecular Formula | C15H24 | |
| IUPAC Name* |
(1aR,4R,4aR,7bS)-1,1,4,7-tetramethyl-1a,2,3,4,4a,5,6,7b-octahydrocyclopropa[e]azulene
|
|
| SMILES |
C[C@@H]1CC[C@@H]2[C@@H](C2(C)C)C3=C(CC[C@H]13)C
|
|
| InChI |
InChI=1S/C15H24/c1-9-6-8-12-14(15(12,3)4)13-10(2)5-7-11(9)13/h9,11-12,14H,5-8H2,1-4H3/t9-,11-,12-,14-/m1/s1
|
|
| InChIKey |
SPCXZDDGSGTVAW-XIDUGBJDSA-N
|
|
| Synonyms |
alpha-Gurjunene; (-)-alpha-Gurjunene; 489-40-7; (1aR,4R,4aR,7bS)-1,1,4,7-tetramethyl-1a,2,3,4,4a,5,6,7b-octahydro-1H-cyclopropa[e]azulene; alpha-Grujunene; Gurjunene-alpha; DTXSID0052126; CHEBI:61699; ZINC59200506; C19734; Q27131296; (1aR,4R,4aR,7bS)-1,1,4,7-tetramethyl-1a,2,3,4,4a,5,6,7b-octahydrocyclopropa[e]azulene
|
|
| CAS | 489-40-7 | |
| PubChem CID | 15560276 | |
| ChEMBL ID | NA |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 204.35 | ALogp: | 4.1 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 0 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 3 |
| Heavy Atoms: | 15 | QED Weighted: | 0.491 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.585 | MDCK Permeability: | 0.00001880 |
| Pgp-inhibitor: | 0.124 | Pgp-substrate: | 0.002 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.511 |
| 30% Bioavailability (F30%): | 0.486 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.132 | Plasma Protein Binding (PPB): | 97.57% |
| Volume Distribution (VD): | 5.067 | Fu: | 1.91% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.723 | CYP1A2-substrate: | 0.592 |
| CYP2C19-inhibitor: | 0.471 | CYP2C19-substrate: | 0.894 |
| CYP2C9-inhibitor: | 0.548 | CYP2C9-substrate: | 0.389 |
| CYP2D6-inhibitor: | 0.033 | CYP2D6-substrate: | 0.144 |
| CYP3A4-inhibitor: | 0.478 | CYP3A4-substrate: | 0.421 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.203 | Half-life (T1/2): | 0.089 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.011 | Human Hepatotoxicity (H-HT): | 0.207 |
| Drug-inuced Liver Injury (DILI): | 0.593 | AMES Toxicity: | 0.003 |
| Rat Oral Acute Toxicity: | 0.317 | Maximum Recommended Daily Dose: | 0.125 |
| Skin Sensitization: | 0.107 | Carcinogencity: | 0.036 |
| Eye Corrosion: | 0.939 | Eye Irritation: | 0.917 |
| Respiratory Toxicity: | 0.712 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC003084 |  |
0.439 | D0S3WH |  |
0.293 | ||
| ENC002222 | 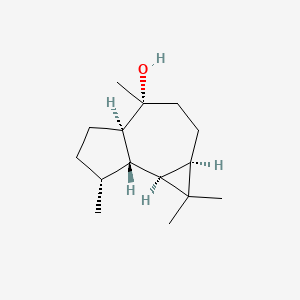 |
0.400 | D0N6FH |  |
0.276 | ||
| ENC002374 |  |
0.390 | D00YWP |  |
0.273 | ||
| ENC001408 | 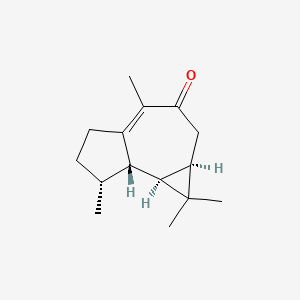 |
0.377 | D0Y5ZA |  |
0.272 | ||
| ENC003074 |  |
0.377 | D04SFH | 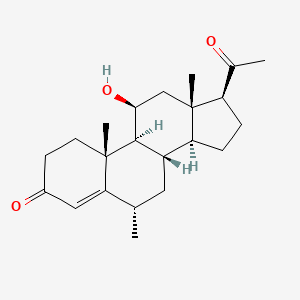 |
0.267 | ||
| ENC001321 |  |
0.367 | D06XMU |  |
0.266 | ||
| ENC000808 |  |
0.367 | D07BSQ |  |
0.265 | ||
| ENC003090 |  |
0.367 | D0F1UL |  |
0.265 | ||
| ENC003089 |  |
0.359 | D0V2JK |  |
0.264 | ||
| ENC001192 |  |
0.349 | D08QMX | 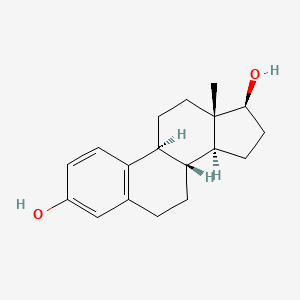 |
0.256 | ||