NPs Basic Information
|
Name |
Luteolin
|
| Molecular Formula | C15H10O6 | |
| IUPAC Name* |
2-(3,4-dihydroxyphenyl)-5,7-dihydroxychromen-4-one
|
|
| SMILES |
C1=CC(=C(C=C1C2=CC(=O)C3=C(C=C(C=C3O2)O)O)O)O
|
|
| InChI |
InChI=1S/C15H10O6/c16-8-4-11(19)15-12(20)6-13(21-14(15)5-8)7-1-2-9(17)10(18)3-7/h1-6,16-19H
|
|
| InChIKey |
IQPNAANSBPBGFQ-UHFFFAOYSA-N
|
|
| Synonyms |
luteolin; 491-70-3; Digitoflavone; Luteolol; 3',4',5,7-Tetrahydroxyflavone; 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4-one; Flacitran; Luteoline; Salifazide; Weld Lake; Yama kariyasu; Cyanidenon 1470; Bismite; 5,7,3',4'-Tetrahydroxyflavone; 2-(3,4-dihydroxyphenyl)-5,7-dihydroxychromen-4-one; C.I. Natural Yellow 2; 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-benzopyrone; Daphneflavonol; Flavopurpol; 4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-; Cyanidenon-1470; BRN 0292084; MFCD00017309; KUX1ZNC9J2; CHEMBL151; FLAVONE, 3',4',5,7-TETRAHYDROXY-; CHEBI:15864; 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-1-benzopyran-4-one; 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-chromenone; 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-chromen-4-one; 1318-21-4; SMR000326896; 7-Tetrahydroxyflavone; CCRIS 3790; SR-01000779333; EINECS 207-741-0; UNII-KUX1ZNC9J2; Argemexitin; C.I. 75590; 4dew; 4dgn; 4hkn; Luteolin,(S); LU2; Prestwick_122; LUTEOLIN [INCI]; LUTEOLIN [MI]; Prestwick0_000870; Prestwick1_000870; Prestwick2_000870; Prestwick3_000870; LUTEOLIN [WHO-DD]; BIDD:PXR0059; Lopac0_000660; Oprea1_849964; SCHEMBL20426; BSPBio_000919; Luteolin, analytical standard; MLS000697655; MLS000860038; MLS002154043; MLS006011917; BIDD:ER0122; SPBio_002840; BDBM7459; BPBio1_001011; GTPL5215; MEGxp0_000143; DTXSID4074988; ACon1_000223; cid_5280445; HMS1570N21; HMS2097N21; HMS2220C06; HMS3356L02; HMS3561N09; HMS3649N21; HMS3656A05; HMS3714N21; Luteolin, >=99.0% (TLC); BCP03511; HY-N0162; TNP00073; Luteolin, >=98% (TLC), powder; BBL027837; LMPK12110006; s2320; STK801923; ZINC18185774; AKOS002140588; AC-1125; BCP9000865; CCG-208309; CS-4611; DB15584; KS-5202; Luteolin 100 microg/mL in Acetonitrile; SMP2_000042; NCGC00016467-01; NCGC00016467-02; NCGC00016467-03; NCGC00016467-04; NCGC00016467-05; NCGC00016467-06; NCGC00016467-07; NCGC00016467-08; NCGC00016467-21; NCGC00142375-01; NCGC00142375-02; NCGC00142375-03; NCGC00179375-01; NCGC00179375-02; CAS-491-70-3; SY030155; BCP0726000198; FT-0600053; SW196433-3; T2682; 91L703; C01514; L 9283; S00110; EN300-1659559; A827664; Luteolin, primary pharmaceutical reference standard; Q415011; Q-100551; SR-01000779333-4; SR-01000779333-5; SR-01000779333-7; BRD-K05236810-001-05-9; 23A002A4-B47B-46CD-848C-65042EACF3FF; NCGC00142375-01,NCGC00142375-02; Z1741977179; 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4H-benzopyrone-4-one; 2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4-one #; 4H-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-
|
|
| CAS | 491-70-3 | |
| PubChem CID | 5280445 | |
| ChEMBL ID | CHEMBL151 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 286.24 | ALogp: | 1.4 |
| HBD: | 4 | HBA: | 6 |
| Rotatable Bonds: | 1 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 107.0 | Aromatic Rings: | 3 |
| Heavy Atoms: | 21 | QED Weighted: | 0.512 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.028 | MDCK Permeability: | 0.00001000 |
| Pgp-inhibitor: | 0.004 | Pgp-substrate: | 0.274 |
| Human Intestinal Absorption (HIA): | 0.047 | 20% Bioavailability (F20%): | 0.998 |
| 30% Bioavailability (F30%): | 1 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.009 | Plasma Protein Binding (PPB): | 95.44% |
| Volume Distribution (VD): | 0.533 | Fu: | 5.98% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.981 | CYP1A2-substrate: | 0.154 |
| CYP2C19-inhibitor: | 0.124 | CYP2C19-substrate: | 0.046 |
| CYP2C9-inhibitor: | 0.576 | CYP2C9-substrate: | 0.842 |
| CYP2D6-inhibitor: | 0.568 | CYP2D6-substrate: | 0.559 |
| CYP3A4-inhibitor: | 0.549 | CYP3A4-substrate: | 0.092 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 8.146 | Half-life (T1/2): | 0.898 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.064 | Human Hepatotoxicity (H-HT): | 0.084 |
| Drug-inuced Liver Injury (DILI): | 0.905 | AMES Toxicity: | 0.536 |
| Rat Oral Acute Toxicity: | 0.046 | Maximum Recommended Daily Dose: | 0.741 |
| Skin Sensitization: | 0.946 | Carcinogencity: | 0.095 |
| Eye Corrosion: | 0.009 | Eye Irritation: | 0.944 |
| Respiratory Toxicity: | 0.22 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
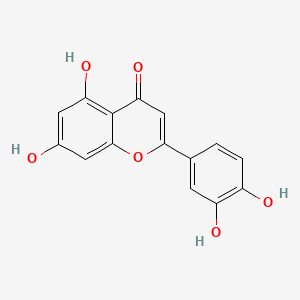
| ENC001533 |  |
0.701 | D04AIT | 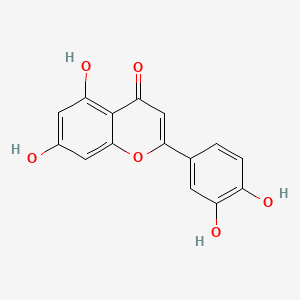 |
1.000 | ||
| ENC001529 |  |
0.686 | D0K8KX | 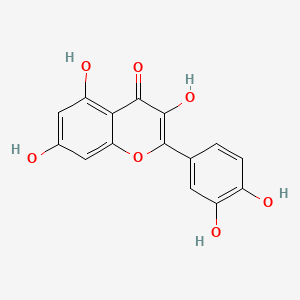 |
0.686 | ||
| ENC001548 |  |
0.568 | D06GCK | 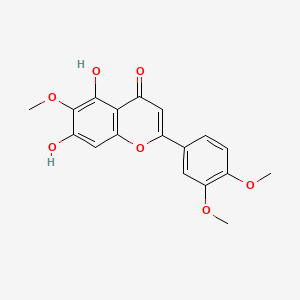 |
0.460 | ||
| ENC001751 |  |
0.550 | D07MGA |  |
0.368 | ||
| ENC004389 | 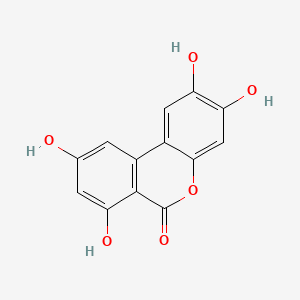 |
0.549 | D0U3YB | 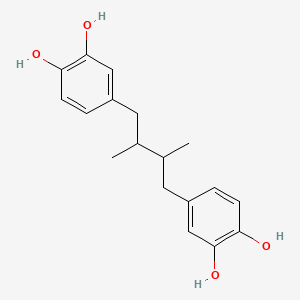 |
0.341 | ||
| ENC001573 |  |
0.545 | D0TC7C | 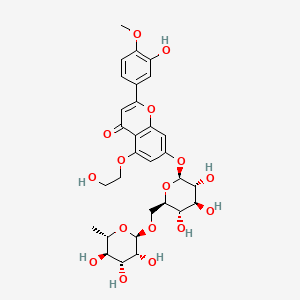 |
0.312 | ||
| ENC001575 |  |
0.495 | D0AZ8C | 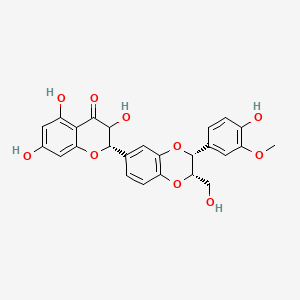 |
0.311 | ||
| ENC005391 |  |
0.494 | D08LFZ | 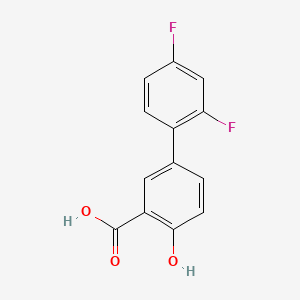 |
0.289 | ||
| ENC002024 | 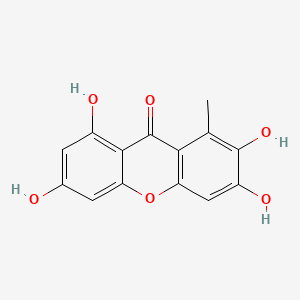 |
0.493 | D00KRE | 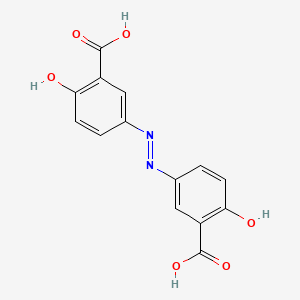 |
0.283 | ||
| ENC002018 | 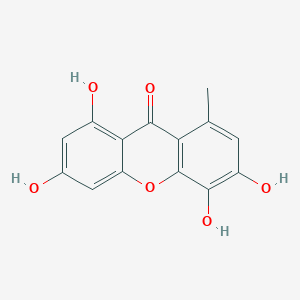 |
0.493 | D06KYN |  |
0.281 | ||