NPs Basic Information
|
Name |
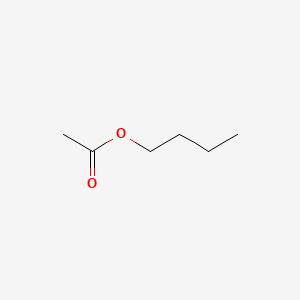
Butyl acetate
|
| Molecular Formula | C6H12O2 | |
| IUPAC Name* |
butyl acetate
|
|
| SMILES |
CCCCOC(=O)C
|
|
| InChI |
InChI=1S/C6H12O2/c1-3-4-5-8-6(2)7/h3-5H2,1-2H3
|
|
| InChIKey |
DKPFZGUDAPQIHT-UHFFFAOYSA-N
|
|
| Synonyms |
Butyl acetate; N-BUTYL ACETATE; 123-86-4; Acetic acid, butyl ester; Butyl ethanoate; 1-Butyl acetate; Acetic Acid Butyl Ester; n-Butylacetate; n-Butyl ethanoate; Butylacetat; Acetic acid n-butyl ester; Acetate de butyle; Butylacetaten; 1-acetoxybutane; Octan n-butylu; Butyle (acetate de); Butylester kyseliny octove; Butyl ester of acetic acid; NSC 9298; Butile(acetati di); 1-Butanol, acetate; MFCD00009445; CH3COO(CH2)3CH3; n-Butyl acetate, HPLC Grade; CHEBI:31328; NSC-9298; 1-Butylacetate; 464P5N1905; Butyl acetate, n-; Butylacetat [German]; Butylacetaten [Dutch]; Octan n-butylu [Polish]; n-Butyl acetate (natural); Acetate de butyle [French]; Butyle (acetate de) [French]; Butile (acetati di); CCRIS 2287; HSDB 152; Butile (acetati di) [Italian]; Butylester kyseliny octove [Czech]; EINECS 204-658-1; BRN 1741921; ACETIC ACID,BUTYL ESTER; AI3-00406; nBuOAc; acetic acid butyl; AcOBu; BuOAc; UNII-464P5N1905; n-BuOAc; Butyle(acetate de); Essigsaeurebutylester; normal-butyl acetate; Nat. Butyl Acetate; Essigsaeure-n-butylester; Butyl ester, acetic acid; DSSTox_CID_1982; EC 204-658-1; Acetic acid, n-butyl ester; BUTYL ACETATE [FCC]; DSSTox_RID_76441; DSSTox_GSID_21982; SCHEMBL14969; BUTYL ACETATE [FHFI]; BUTYL ACETATE [INCI]; 4-02-00-00143 (Beilstein Handbook Reference); WLN: 4OV1; N-BUTYL ACETATE [MI]; n-Butyl acetate, ACS reagent; BUTYL ACETATE [MART.]; BUTYL ESTER ACETIC ACID; Butyl acetate, AR, 99.5%; Butyl acetate, LR, >=98%; CHEMBL284391; BUTYL ACETATE [USP-RS]; N-BUTYL ACETATE [HSDB]; DTXSID3021982; FEMA NO. 2174; Butyl Acetate (Fragrance Grade); Butyl Acetate Reagent ACS Grade; Butyl Acetate (Industrial Grade); n-Butyl acetate Biochemical grade; NSC9298; Butyl acetate, ampule of 100 mg; Butyl acetate, analytical standard; Butyl acetate, anhydrous, >=99%; AMY11075; ZINC1699905; Butyl acetate, for HPLC, 99.7%; n-Butyl acetate, analytical standard; Tox21_201052; n-Butyl acetate, Semiconductor Grade; STL282735; Butyl acetate, >=99%, FCC, FG; AKOS000120198; Butyl acetate, natural, >=98%, FG; Butyl acetate, ACS reagent, >=99.5%; Butyl acetate, ReagentPlus(R), 99.5%; NCGC00091573-01; NCGC00091573-02; NCGC00258605-01; CAS-123-86-4; Butyl acetate, puriss. p.a., ACS reagent; A0024; A0228; Butyl acetate, SAJ first grade, >=98.0%; FT-0621752; Butyl acetate, JIS special grade, >=99.0%; EN300-21265; n-Butylacetate 100 microg/mL in Acetonitrile; n-Butyl acetate [UN1123] [Flammable liquid]; A805161; Q411073; J-004991; J-519958; Q-200771; TRIBUTYL ACETYLCITRATE IMPURITY E [EP IMPURITY]; F0001-0371; Butyl acetate, >=99.5%, suitable for atomic absorption spectrometry; Butyl acetate, United States Pharmacopeia (USP) Reference Standard; Butyl acetate, Pharmaceutical Secondary Standard; Certified Reference Material; 8JZ
|
|
| CAS | 123-86-4 | |
| PubChem CID | 31272 | |
| ChEMBL ID | CHEMBL284391 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 116.16 | ALogp: | 1.8 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 4 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 26.3 | Aromatic Rings: | 0 |
| Heavy Atoms: | 8 | QED Weighted: | 0.415 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.215 | MDCK Permeability: | 0.00003090 |
| Pgp-inhibitor: | 0.001 | Pgp-substrate: | 0.005 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.812 |
| 30% Bioavailability (F30%): | 0.946 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.997 | Plasma Protein Binding (PPB): | 37.46% |
| Volume Distribution (VD): | 0.906 | Fu: | 76.85% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.939 | CYP1A2-substrate: | 0.278 |
| CYP2C19-inhibitor: | 0.256 | CYP2C19-substrate: | 0.684 |
| CYP2C9-inhibitor: | 0.052 | CYP2C9-substrate: | 0.226 |
| CYP2D6-inhibitor: | 0.037 | CYP2D6-substrate: | 0.249 |
| CYP3A4-inhibitor: | 0.019 | CYP3A4-substrate: | 0.254 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.221 | Half-life (T1/2): | 0.778 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.028 | Human Hepatotoxicity (H-HT): | 0.018 |
| Drug-inuced Liver Injury (DILI): | 0.229 | AMES Toxicity: | 0.012 |
| Rat Oral Acute Toxicity: | 0.03 | Maximum Recommended Daily Dose: | 0.015 |
| Skin Sensitization: | 0.554 | Carcinogencity: | 0.272 |
| Eye Corrosion: | 0.977 | Eye Irritation: | 0.991 |
| Respiratory Toxicity: | 0.095 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000264 |  |
0.645 | D0Q9HF |  |
0.438 | ||
| ENC000188 |  |
0.567 | D01QLH |  |
0.344 | ||
| ENC000245 |  |
0.548 | D0ZK8H |  |
0.323 | ||
| ENC000377 | 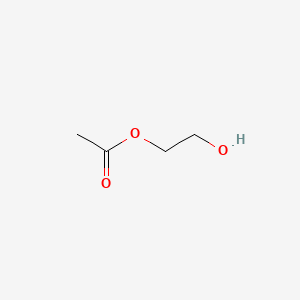 |
0.500 | D0AY9Q |  |
0.313 | ||
| ENC000605 | 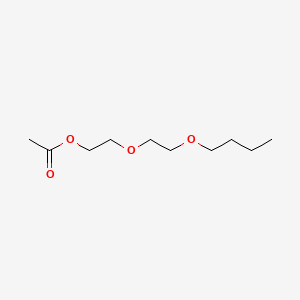 |
0.500 | D0U7BW | 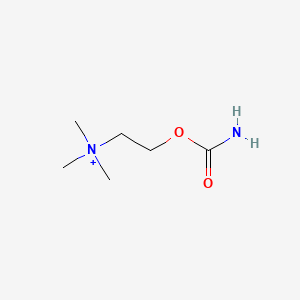 |
0.278 | ||
| ENC000312 |  |
0.500 | D0Y3KG |  |
0.270 | ||
| ENC000211 |  |
0.472 | D02HXS |  |
0.269 | ||
| ENC000726 | 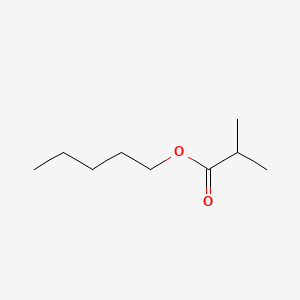 |
0.471 | D08HQK |  |
0.254 | ||
| ENC000849 |  |
0.469 | D0Y4AW |  |
0.250 | ||
| ENC000603 |  |
0.467 | D0Q6DX |  |
0.250 | ||