NPs Basic Information

|
Name |
Heptadecanoic acid
|
| Molecular Formula | C17H34O2 | |
| IUPAC Name* |
heptadecanoic acid
|
|
| SMILES |
CCCCCCCCCCCCCCCCC(=O)O
|
|
| InChI |
InChI=1S/C17H34O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17(18)19/h2-16H2,1H3,(H,18,19)
|
|
| InChIKey |
KEMQGTRYUADPNZ-UHFFFAOYSA-N
|
|
| Synonyms |
HEPTADECANOIC ACID; Margaric acid; 506-12-7; n-Heptadecanoic acid; Margarinic acid; n-Heptadecylic acid; Heptadecylic acid; n-Heptadecoic acid; C17:0; Normal-heptadecanoic acid; MFCD00002751; V987Y9OZ8L; CHEBI:32365; margarinate; margaroate; NSC-3743; n-heptadecoate; n-heptadecylate; 68424-37-3; NSC 3743; Lead(2+) heptadecanoate; 63399-94-0; EINECS 208-027-1; BRN 1781004; UNII-V987Y9OZ8L; Margarinsaeure; margaric'acid; margaroic acid; Daturinic acid; Daturic acid; heptadecoic acid; AI3-36481; Heptadecanoic acic; X90; EINECS 270-298-7; Normal-heptadecanoate; heptadecansäure; Heptadecanoic acid, 98%; DSSTox_CID_1596; EC 270-298-7; MARGARIC ACID [MI]; SCHEMBL5941; DSSTox_RID_78651; DSSTox_GSID_28306; Heptadecanoic acid, >=98%; 4-02-00-01193 (Beilstein Handbook Reference); WLN: QV16; CH3-[CH2]15-COOH; CHEMBL1172910; DTXSID5021596; FA 17:0a; AMY5932; NSC3743; EINECS 264-123-3; EINECS 266-928-5; Tox21_202550; BBL013181; LMFA01010017; s3336; STL163960; ZINC24805047; AKOS005716689; CS-W004284; HY-W004284; NSC 122836; NCGC00260099-01; AS-57021; BP-27918; BP-30092; SY010570; SDA 19-005-00; CAS-67701-03-5; FT-0628169; H0019; EN300-19173; EC 266-928-5; H10748; A871505; Q902204; 8EBAABCC-09B2-43FB-945D-A70E5905BFBE; Z104473032
|
|
| CAS | 506-12-7 | |
| PubChem CID | 10465 | |
| ChEMBL ID | CHEMBL1172910 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 270.5 | ALogp: | 6.9 |
| HBD: | 1 | HBA: | 2 |
| Rotatable Bonds: | 15 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 37.3 | Aromatic Rings: | 0 |
| Heavy Atoms: | 19 | QED Weighted: | 0.369 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -5.053 | MDCK Permeability: | 0.00002280 |
| Pgp-inhibitor: | 0.005 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.591 |
| 30% Bioavailability (F30%): | 0.993 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.042 | Plasma Protein Binding (PPB): | 99.20% |
| Volume Distribution (VD): | 0.69 | Fu: | 0.94% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.293 | CYP1A2-substrate: | 0.189 |
| CYP2C19-inhibitor: | 0.247 | CYP2C19-substrate: | 0.077 |
| CYP2C9-inhibitor: | 0.146 | CYP2C9-substrate: | 0.99 |
| CYP2D6-inhibitor: | 0.009 | CYP2D6-substrate: | 0.048 |
| CYP3A4-inhibitor: | 0.029 | CYP3A4-substrate: | 0.016 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 2.399 | Half-life (T1/2): | 0.542 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.065 | Human Hepatotoxicity (H-HT): | 0.024 |
| Drug-inuced Liver Injury (DILI): | 0.043 | AMES Toxicity: | 0.005 |
| Rat Oral Acute Toxicity: | 0.026 | Maximum Recommended Daily Dose: | 0.015 |
| Skin Sensitization: | 0.92 | Carcinogencity: | 0.058 |
| Eye Corrosion: | 0.977 | Eye Irritation: | 0.972 |
| Respiratory Toxicity: | 0.901 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000110 |  |
0.947 | D07ILQ |  |
0.727 | ||
| ENC000050 |  |
0.944 | D0O1PH |  |
0.622 | ||
| ENC000466 |  |
0.889 | D00AOJ |  |
0.592 | ||
| ENC000357 | 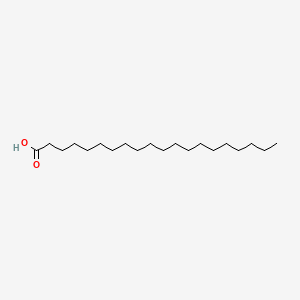 |
0.857 | D0Z5SM | 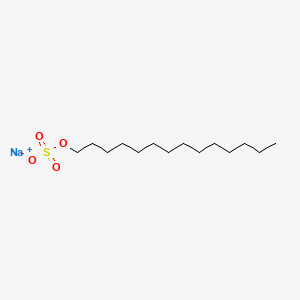 |
0.565 | ||
| ENC000378 |  |
0.833 | D00FGR |  |
0.536 | ||
| ENC000496 | 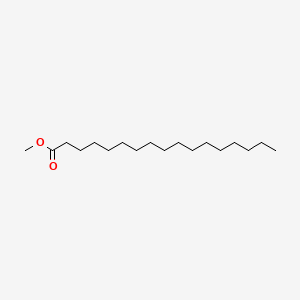 |
0.790 | D05ATI | 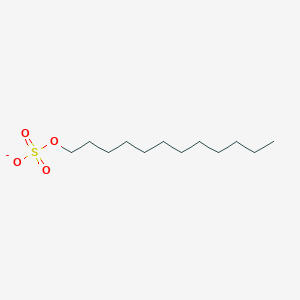 |
0.485 | ||
| ENC000608 | 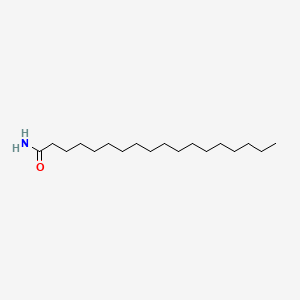 |
0.790 | D0XN8C |  |
0.468 | ||
| ENC000282 | 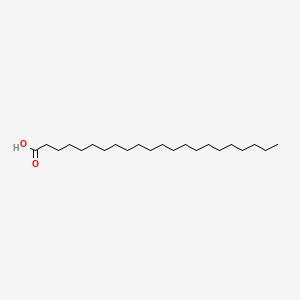 |
0.783 | D0Z5BC |  |
0.452 | ||
| ENC000688 |  |
0.780 | D0P1RL |  |
0.437 | ||
| ENC000082 | 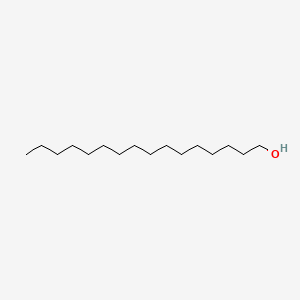 |
0.776 | D0O1TC |  |
0.427 | ||