NPs Basic Information
|
Name |
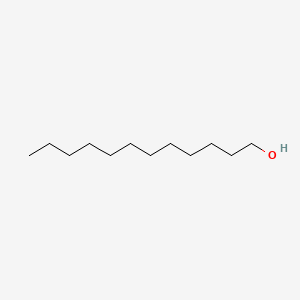
1-Dodecanol
|
| Molecular Formula | C12H26O | |
| IUPAC Name* |
dodecan-1-ol
|
|
| SMILES |
CCCCCCCCCCCCO
|
|
| InChI |
InChI=1S/C12H26O/c1-2-3-4-5-6-7-8-9-10-11-12-13/h13H,2-12H2,1H3
|
|
| InChIKey |
LQZZUXJYWNFBMV-UHFFFAOYSA-N
|
|
| Synonyms |
1-DODECANOL; Dodecan-1-ol; Dodecyl alcohol; Dodecanol; Lauryl alcohol; 112-53-8; n-Dodecyl alcohol; Undecyl carbinol; Dodecylalcohol; Lauric alcohol; Laurinic alcohol; 1-Dodecyl alcohol; Pisol; n-Dodecan-1-ol; Duodecyl alcohol; 1-Hydroxydodecane; Siponol L5; Karukoru 20; Lauroyl alcohol; Siponol 25; Lorol 5; Lorol 7; n-Dodecanol; Lauryl 24; Alcohol C-12; Alfol 12; Lorol 11; Sipol L12; Dytol J-68; Siponol L2; Cachalot L-50; Cachalot L-90; Dodecyl alcoho; n-Lauryl alcohol; C12 alcohol; Hainol 12SS; Hydroxydodecane; Conol 20P; Conol 20PP; Lorol; EPAL 12; Adol 10; Adol 12; Dodecanol-1; n-Lauryl alcohol, primary; Nacol 12-96; Alcohol C12; FEMA No. 2617; NAA 42; CO-1214; Lipocol L; CO-1214N; CO-1214S; MFCD00004753; S 1298; MA-1214; Lorol C12; Co-1214S1-dodecanol; 27342-88-7; 68551-07-5; CHEBI:28878; 178A96NLP2; NSC-3724; DSSTox_CID_6918; DSSTox_RID_78253; DSSTox_GSID_26918; Adol 11; Lorol C 12; FEMA Number 2617; Dytol J-68 (VAN); Lorol C 12/98; 1DO; CAS-112-53-8; CCRIS 662; Dodecanol, 1-; HSDB 1075; NSC 3724; EINECS 203-982-0; BRN 1738860; laurylalcohol; Lorol special; UNII-178A96NLP2; AI3-00309; EINECS 271-359-0; Philcohol 1200; LAUREX NC; LAUREX L1; 1-DODECANOL [MI]; 1-Dodecanol, 98.0%; EC 203-982-0; SCHEMBL6844; 1-DODECANOL [HSDB]; LAURYL ALCOHOL [FCC]; 4-01-00-01844 (Beilstein Handbook Reference); CHEMBL24722; LAURYL ALCOHOL [FHFI]; LAURYL ALCOHOL [INCI]; C12H25OH; WLN: Q12; Lauryl alcohol, >=98%, FG; NACOL 12-99 ALCOHOL; DTXSID5026918; LAURYL ALCOHOL [USP-RS]; 1-dodecanol (ACD/Name 4.0); 1-Dodecanol, analytical standard; ALFOL 1216 CO ALCOHOL; NSC3724; 1-Dodecanol, reagent grade, 98%; BCP29203; CS-D1360; HY-Y0289; ZINC1529403; Tox21_202124; Tox21_300120; LMFA05000001; STL301829; CACHALOT L-90 LAURYL ALCOHOL; Co 12Co-1214Co-1214N; AKOS009031450; DB06894; 1-Dodecanol, ACS reagent, >=98.0%; NCGC00164341-01; NCGC00164341-02; NCGC00164341-03; NCGC00253987-01; NCGC00259673-01; CS-16955; 1-Dodecanol 100 microg/mL in Acetonitrile; DB-003637; 1-Dodecanol, SAJ special grade, >=97.0%; 1-Dodecanol, Selectophore(TM), >=98.0%; 1-dodecanol; dodecyl alcohol; lauryl alcohol; D0978; FT-0607710; FT-0693265; 1-Dodecanol, Vetec(TM) reagent grade, 98%; EN300-20043; C02277; Q161617; Q-200121; Dodecan-1-ol;Dodecyl alcohol;Lauryl alcohol;Dodecanol; Z104476554; Lauryl alcohol, United States Pharmacopeia (USP) Reference Standard
|
|
| CAS | 112-53-8 | |
| PubChem CID | 8193 | |
| ChEMBL ID | CHEMBL24722 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 186.33 | ALogp: | 5.1 |
| HBD: | 1 | HBA: | 1 |
| Rotatable Bonds: | 10 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 20.2 | Aromatic Rings: | 0 |
| Heavy Atoms: | 13 | QED Weighted: | 0.496 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.483 | MDCK Permeability: | 0.00002070 |
| Pgp-inhibitor: | 0.005 | Pgp-substrate: | 0.008 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.25 |
| 30% Bioavailability (F30%): | 0.983 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.769 | Plasma Protein Binding (PPB): | 95.25% |
| Volume Distribution (VD): | 1.863 | Fu: | 3.35% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.87 | CYP1A2-substrate: | 0.244 |
| CYP2C19-inhibitor: | 0.43 | CYP2C19-substrate: | 0.065 |
| CYP2C9-inhibitor: | 0.275 | CYP2C9-substrate: | 0.888 |
| CYP2D6-inhibitor: | 0.012 | CYP2D6-substrate: | 0.07 |
| CYP3A4-inhibitor: | 0.082 | CYP3A4-substrate: | 0.066 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 7.369 | Half-life (T1/2): | 0.325 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.125 | Human Hepatotoxicity (H-HT): | 0.015 |
| Drug-inuced Liver Injury (DILI): | 0.042 | AMES Toxicity: | 0.007 |
| Rat Oral Acute Toxicity: | 0.036 | Maximum Recommended Daily Dose: | 0.013 |
| Skin Sensitization: | 0.934 | Carcinogencity: | 0.061 |
| Eye Corrosion: | 0.991 | Eye Irritation: | 0.965 |
| Respiratory Toxicity: | 0.396 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000274 | 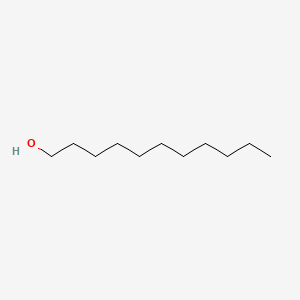 |
0.919 | D05ATI | 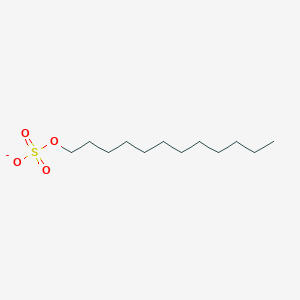 |
0.615 | ||
| ENC000426 | 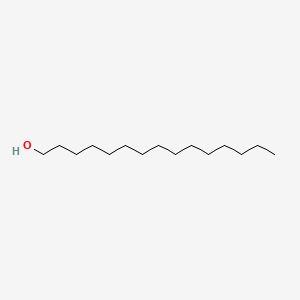 |
0.804 | D07ILQ |  |
0.565 | ||
| ENC000327 |  |
0.762 | D00AOJ |  |
0.552 | ||
| ENC000421 |  |
0.762 | D0Z5SM |  |
0.542 | ||
| ENC000317 |  |
0.757 | D0O1PH |  |
0.493 | ||
| ENC000082 |  |
0.755 | D05QNO |  |
0.417 | ||
| ENC000486 |  |
0.712 | D0MM8N |  |
0.411 | ||
| ENC000422 |  |
0.711 | D00FGR |  |
0.400 | ||
| ENC001240 |  |
0.711 | D0Y8DP |  |
0.393 | ||
| ENC000475 |  |
0.711 | D0XN8C |  |
0.391 | ||