NPs Basic Information

|
Name |
1-Dodecene
|
| Molecular Formula | C12H24 | |
| IUPAC Name* |
dodec-1-ene
|
|
| SMILES |
CCCCCCCCCCC=C
|
|
| InChI |
InChI=1S/C12H24/c1-3-5-7-9-11-12-10-8-6-4-2/h3H,1,4-12H2,2H3
|
|
| InChIKey |
CRSBERNSMYQZNG-UHFFFAOYSA-N
|
|
| Synonyms |
1-DODECENE; Dodec-1-ene; 112-41-4; DODECENE; Adacene 12; Dodecene-1; Dodecylene; n-Dodec-1-ene; .alpha.-Dodecene; 25378-22-7; .alpha.-Dodecylene; NSC 12016; Dodecylene .alpha.-; 1-Dodecene, dimer; MFCD00008961; WYE669F3GR; CHEBI:89713; NSC-12016; 62132-67-6; 1-Dodecene, 95%; alpha-Dodecene; alpha-Dodecylene; DSSTox_CID_6914; DSSTox_RID_78251; DSSTox_GSID_26914; N-Dodec-1-Ene A-Dodecylene; 68526-58-9; CAS-112-41-4; HSDB 1076; EINECS 203-968-4; UNII-WYE669F3GR; n-dodecene; HSDB 2793; dodec-11-ene; Neodene 12; EINECS 246-922-9; DODECENE [INCI]; DODECENE, 1-; EC 203-968-4; EC 246-922-9; 1-DODECENE [HSDB]; 1-Dodecene (standard material); 1-Dodecene, analytical standard; CHEMBL1872885; DTXSID5026914; NSC12016; ZINC1718715; EINECS 271-215-7; Tox21_201382; Tox21_303303; 1-Dodecene, >=99.0% (GC); LMFA11000313; AKOS015904161; CS-W017788; 1-Dodecene, technical, >=90% (GC); NCGC00164290-01; NCGC00164290-02; NCGC00257096-01; NCGC00258933-01; BS-14425; DB-041090; D0974; FT-0607712; S0342; EN300-99514; D70997; A802575; Q161620; J-002769
|
|
| CAS | 112-41-4 | |
| PubChem CID | 8183 | |
| ChEMBL ID | CHEMBL1872885 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 168.32 | ALogp: | 6.8 |
| HBD: | 0 | HBA: | 0 |
| Rotatable Bonds: | 9 | Lipinski's rule of five: | Rejected |
| Polar Surface Area: | 0.0 | Aromatic Rings: | 0 |
| Heavy Atoms: | 12 | QED Weighted: | 0.33 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.547 | MDCK Permeability: | 0.00001490 |
| Pgp-inhibitor: | 0.02 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.28 |
| 30% Bioavailability (F30%): | 0.633 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.952 | Plasma Protein Binding (PPB): | 97.94% |
| Volume Distribution (VD): | 1.56 | Fu: | 2.57% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.904 | CYP1A2-substrate: | 0.311 |
| CYP2C19-inhibitor: | 0.613 | CYP2C19-substrate: | 0.239 |
| CYP2C9-inhibitor: | 0.34 | CYP2C9-substrate: | 0.918 |
| CYP2D6-inhibitor: | 0.146 | CYP2D6-substrate: | 0.531 |
| CYP3A4-inhibitor: | 0.522 | CYP3A4-substrate: | 0.105 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 4.926 | Half-life (T1/2): | 0.18 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.055 | Human Hepatotoxicity (H-HT): | 0.012 |
| Drug-inuced Liver Injury (DILI): | 0.038 | AMES Toxicity: | 0.013 |
| Rat Oral Acute Toxicity: | 0.036 | Maximum Recommended Daily Dose: | 0.031 |
| Skin Sensitization: | 0.952 | Carcinogencity: | 0.095 |
| Eye Corrosion: | 0.994 | Eye Irritation: | 0.982 |
| Respiratory Toxicity: | 0.367 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000510 |  |
0.919 | D0Z5BC |  |
0.591 | ||
| ENC000455 |  |
0.912 | D05ATI | 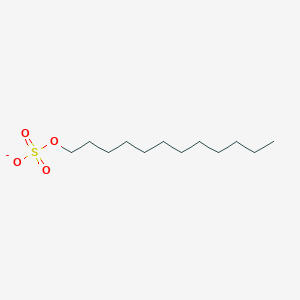 |
0.473 | ||
| ENC000475 | 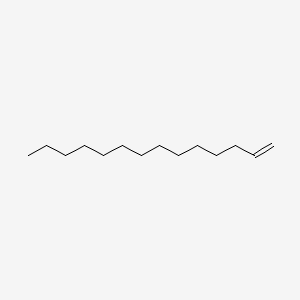 |
0.850 | D0Z5SM | 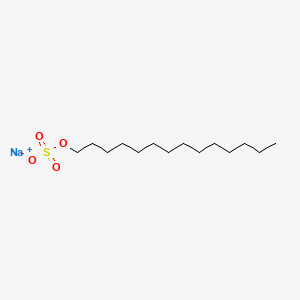 |
0.419 | ||
| ENC000460 | 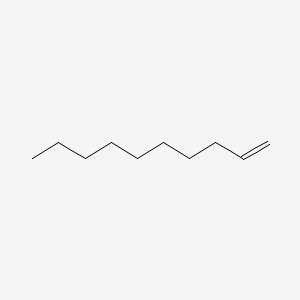 |
0.824 | D0Y8DP |  |
0.415 | ||
| ENC000573 |  |
0.791 | D0O1PH |  |
0.408 | ||
| ENC000275 | 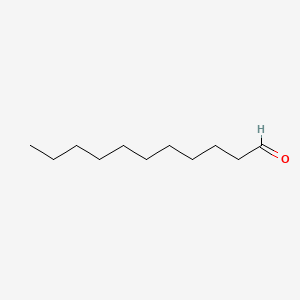 |
0.744 | D07ILQ |  |
0.382 | ||
| ENC000425 |  |
0.739 | D05QNO |  |
0.367 | ||
| ENC000557 | 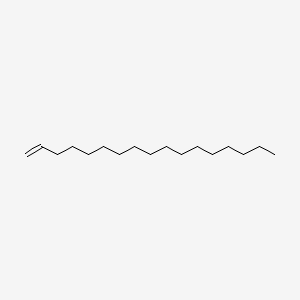 |
0.694 | D03ZJE | 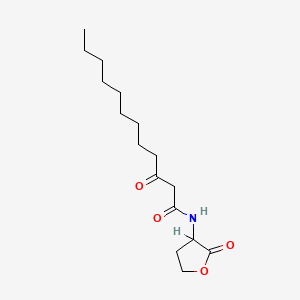 |
0.348 | ||
| ENC000277 | 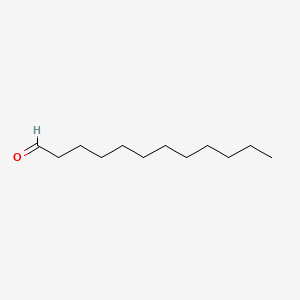 |
0.690 | D0XN8C |  |
0.348 | ||
| ENC001656 |  |
0.690 | D0O1TC |  |
0.347 | ||