NPs Basic Information
|
Name |
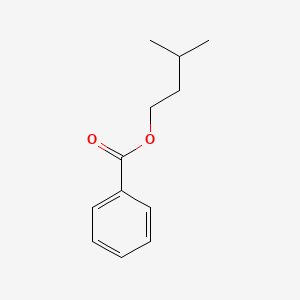
Isopentyl benzoate
|
| Molecular Formula | C12H16O2 | |
| IUPAC Name* |
3-methylbutyl benzoate
|
|
| SMILES |
CC(C)CCOC(=O)C1=CC=CC=C1
|
|
| InChI |
InChI=1S/C12H16O2/c1-10(2)8-9-14-12(13)11-6-4-3-5-7-11/h3-7,10H,8-9H2,1-2H3
|
|
| InChIKey |
MLLAPOCBLWUFAP-UHFFFAOYSA-N
|
|
| Synonyms |
Isoamyl benzoate; Isopentyl benzoate; 94-46-2; 3-Methylbutyl benzoate; Benzoic acid isoamyl ester; Isopentyl alcohol, benzoate; 1-Butanol, 3-methyl-, benzoate; 1-(3-Methyl)butyl benzoate; BENZOIC ACID, ISOPENTYL ESTER; Isoamylbenzoate; 3-Methyl-1-butyl benzoate; Benzoic acid, 1-(3-methyl)butyl ester; FEMA No. 2058; Isoamyl-benzoate; Benzoic acid, 3-methylbutyl ester; NSC 9284; 1-Butanol, 3-methyl-, 1-benzoate; 0AY72CK43K; benzoic acid 3-methylbutyl ester; NSC-9284; Isoamyl benzoate (natural); EINECS 202-334-4; BRN 1946447; UNII-0AY72CK43K; AI3-01966; Isoamyl=benzoate; so-Amyl benzoate; iso-amyl benzoate; 2-Dimethylaminoethylbenzoate (DMB); 1-Butanol, benzoate; Isopentyl alcohol, benzoate (6CI,8CI); DSSTox_CID_27185; DSSTox_RID_82181; DSSTox_GSID_47185; ISOAMYL BENZOATE [MI]; SCHEMBL132524; WLN: 1Y1&2OVR; ISOAMYL BENZOATE [FCC]; ISOAMYL BENZOATE [FHFI]; CHEMBL2260711; DTXSID8047185; FEMA 2058; NSC9284; CHEBI:179910; ZINC394936; 1-Butanol, 3-methyl-,1-benzoate; Benzoic acid-(3-methylbutyl) ester; Tox21_302461; BBL011533; MFCD00026515; STL146651; AKOS005720808; Isoamyl benzoate, >=98%, FCC, FG; CAS-94-46-2; NCGC00256869-01; AC-18094; AS-57016; B0071; CS-0154227; FT-0627319; BENZOIC ACID 1-(3-METHYL)BUTYL ESTER; E75850; A844963; Q27236554; 54846-63-8
|
|
| CAS | 94-46-2 | |
| PubChem CID | 7193 | |
| ChEMBL ID | CHEMBL2260711 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 192.25 | ALogp: | 3.8 |
| HBD: | 0 | HBA: | 2 |
| Rotatable Bonds: | 5 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 26.3 | Aromatic Rings: | 1 |
| Heavy Atoms: | 14 | QED Weighted: | 0.68 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.221 | MDCK Permeability: | 0.00003250 |
| Pgp-inhibitor: | 0.002 | Pgp-substrate: | 0 |
| Human Intestinal Absorption (HIA): | 0.003 | 20% Bioavailability (F20%): | 0.009 |
| 30% Bioavailability (F30%): | 0.965 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.147 | Plasma Protein Binding (PPB): | 96.20% |
| Volume Distribution (VD): | 1.519 | Fu: | 4.07% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.964 | CYP1A2-substrate: | 0.388 |
| CYP2C19-inhibitor: | 0.915 | CYP2C19-substrate: | 0.187 |
| CYP2C9-inhibitor: | 0.819 | CYP2C9-substrate: | 0.699 |
| CYP2D6-inhibitor: | 0.022 | CYP2D6-substrate: | 0.091 |
| CYP3A4-inhibitor: | 0.038 | CYP3A4-substrate: | 0.197 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 12.198 | Half-life (T1/2): | 0.705 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.068 | Human Hepatotoxicity (H-HT): | 0.012 |
| Drug-inuced Liver Injury (DILI): | 0.351 | AMES Toxicity: | 0.006 |
| Rat Oral Acute Toxicity: | 0.011 | Maximum Recommended Daily Dose: | 0.009 |
| Skin Sensitization: | 0.591 | Carcinogencity: | 0.118 |
| Eye Corrosion: | 0.217 | Eye Irritation: | 0.991 |
| Respiratory Toxicity: | 0.102 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000637 |  |
0.744 | D0B7OD | 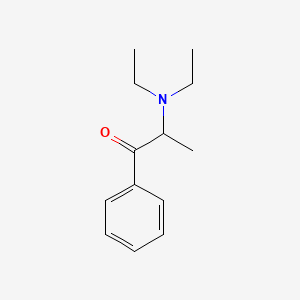 |
0.481 | ||
| ENC000175 | 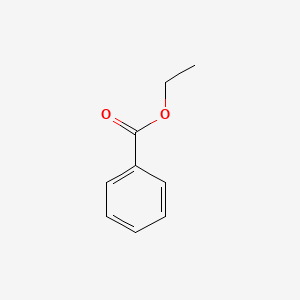 |
0.628 | D0X9RY | 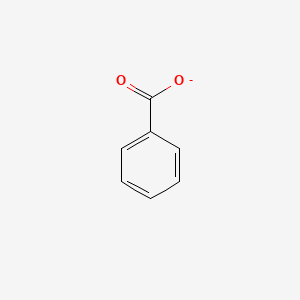 |
0.455 | ||
| ENC001726 |  |
0.577 | D0G1VX | 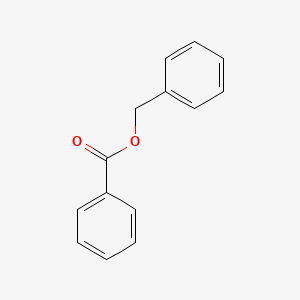 |
0.433 | ||
| ENC000174 |  |
0.558 | D08MRN |  |
0.403 | ||
| ENC000214 | 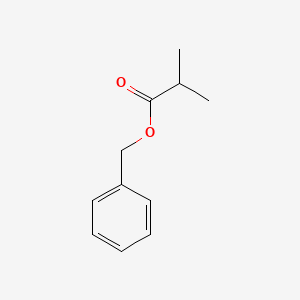 |
0.500 | D02YPG |  |
0.385 | ||
| ENC000651 | 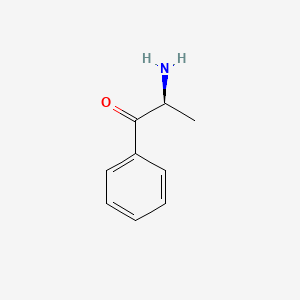 |
0.500 | D0P6UB |  |
0.373 | ||
| ENC000192 |  |
0.488 | D0T3LF |  |
0.367 | ||
| ENC004815 |  |
0.472 | D05BMG |  |
0.367 | ||
| ENC000216 |  |
0.460 | D0R1CR | 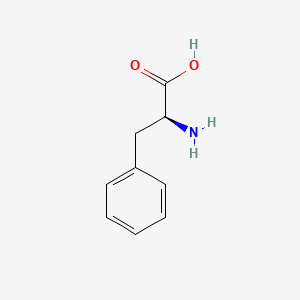 |
0.358 | ||
| ENC000076 |  |
0.455 | D0S7VO | 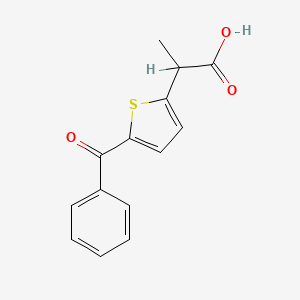 |
0.348 | ||