NPs Basic Information

|
Name |
Vanillin
|
| Molecular Formula | C8H8O3 | |
| IUPAC Name* |
4-hydroxy-3-methoxybenzaldehyde
|
|
| SMILES |
COC1=C(C=CC(=C1)C=O)O
|
|
| InChI |
InChI=1S/C8H8O3/c1-11-8-4-6(5-9)2-3-7(8)10/h2-5,10H,1H3
|
|
| InChIKey |
MWOOGOJBHIARFG-UHFFFAOYSA-N
|
|
| Synonyms |
vanillin; 4-Hydroxy-3-methoxybenzaldehyde; 121-33-5; Vanillaldehyde; Vanillic aldehyde; p-Vanillin; Lioxin; Vanilla; Vanilline; Benzaldehyde, 4-hydroxy-3-methoxy-; 3-Methoxy-4-hydroxybenzaldehyde; 4-Hydroxy-m-anisaldehyde; Zimco; p-Hydroxy-m-methoxybenzaldehyde; 2-Methoxy-4-formylphenol; 4-Hydroxy-3-methoxy-benzaldehyde; Methylprotocatechuic aldehyde; Vanilin; 4-Formyl-2-methoxyphenol; Vanillin (natural); 4-Hydroxy-5-methoxybenzaldehyde; m-Anisaldehyde, 4-hydroxy-; Protocatechualdehyde, methyl-; Rhovanil; FEMA No. 3107; Vanillin (NF); Protocatechualdehyde 3-methyl ether; vaniline; NSC 15351; Vanillin [NF]; m-Methoxy-p-hydroxybenzaldehyde; Vanillin Melting Point Standard; MFCD00006942; NSC-15351; NSC-48383; NSC-403658; CHEMBL13883; CHEBI:18346; NPLC-0145; 4-hydroxy-3-methoxy-benzyldehyde; CHI530446X; 4-HYDROXY,3-METHOXY-BENZALDEHYDE; NCGC00091645-03; 4-hydroxy-3-methoxybenzaldehyde (vanillin); DSSTox_CID_1969; WLN: VHR DQ CO1; H-0264; DSSTox_RID_76433; DSSTox_GSID_21969; Vanillin [USAN]; Oleoresin vanilla; Vanilla oleoresin; MFCD08702848; CAS-121-33-5; CCRIS 2687; HSDB 1027; EINECS 204-465-2; BRN 0472792; UNII-CHI530446X; AI3-00093; Nat.Vanillin; 3-methoxy-4-hydroxy-benzaldehyde; Vanillin, natural; oleo-Resins vanilla; V55; Vanillin sodium salt; VANILLIN [FHFI]; VANILLIN [HSDB]; VANILLIN [INCI]; oleo-Resins vanilla-bean; VANILLIN [FCC]; 4-Hydroxy-3-methoxybenzaldehyde(Vanilline); VANILLIN [II]; VANILLIN [MI]; VANILLIN [VANDF]; Vanillin-[methoxy-13C]; methyl-Protocatechualdehyde; VANILLIN [MART.]; bmse000343; bmse000597; bmse010006; Methylprotcatechuic aldehyde; VANILLIN [USP-RS]; VANILLIN [WHO-DD]; EC 204-465-2; SCHEMBL1213; 4-08-00-01763 (Beilstein Handbook Reference); MLS002303069; BIDD:ER0330; Vanillin, puriss., 99.5%; GTPL6412; SGCUT00016; 4-hydroxy 3-methoxybenzaldehyde; VANILLIN [EP MONOGRAPH]; DTXSID0021969; FEMA 3107; Vanilla oleoresin (vanilla SPP); 3-methoxy-4-hydroxy benzaldehyde; 4-hydroxy-3-methoxy benzaldehyde; VANILLIN, NATURAL [FHFI]; 3-methoxy-4-hydroxy benzoaldehyde; Vanillin, ReagentPlus(R), 99%; 4-hydroxy-3-(methoxy)benzaldehyde; HMS3651D20; HMS3885K07; Vanillin, >=97%, FCC, FG; 4-hydoxy-3-(methyloxy)benzaldehyde; BCP29943; HY-N0098; NSC15351; NSC48383; STR01001; to_000089; ZINC2567933; Tox21_113534; Tox21_201925; Tox21_300352; 4-hydoxy-3-(methyloxy)benz aldehyde; BBL011956; BDBM50177405; METHYL PROTOCATECHUIC ALDEHYDE; NSC403658; s3071; STK199262; AKOS000118929; Tox21_113534_1; CCG-266230; CS-W020052; Vanillin, tested according to Ph.Eur.; NCGC00091645-01; NCGC00091645-02; NCGC00091645-04; NCGC00091645-05; NCGC00091645-07; NCGC00254468-01; NCGC00259474-01; Vanillin, natural, >=97%, FCC, FG; AC-10370; BP-10602; NCI60_001085; SMR000156285; SY224451; Vanillin 1000 microg/mL in Acetonitrile; Vanillin, JIS special grade, >=98.0%; Vanillin, Vetec(TM) reagent grade, 98%; 3-Methoxy-4-hydroxybenzaldehyde (vanillin); DB-003805; AM20060497; FT-0618639; FT-0669738; FT-0675778; H0264; SW219190-1; V0080; EN300-18281; vanillin (3-methoxy-4-hydroxy- benzaldehyde); A19444; C00755; D00091; Q33495; Vanillin (83 degrees C) Melting Point Standard; 4-hydroxy-3-methoxy-Benzaldehyde-5-chlorovanillin; 4-hydroxy-3-methoxybenzaldehyde (ACD/Name 4.0); AC-907/21098004; Q-100102; Vanillin, TraceCERT(R), certified reference material; Z57772449; F2190-0587; Vanillin, European Pharmacopoeia (EP) Reference Standard; 1-(AMINOMETHYL)-CYCLOPROPANECARBOXYLICACIDETHYLESTER; Mettler-Toledo Calibration substance ME 51143093, Vanillin; Vanillin, United States Pharmacopeia (USP) Reference Standard; NSC 15351;NSC-15351; NSC15351 pound>>4-Hydroxy-3-methoxybenzaldehyde; Vanillin, Pharmaceutical Secondary Standard; Certified Reference Material; Vanillin Melting Point Standard, United States Pharmacopeia (USP) Reference Standard; Mettler-Toledo Calibration substance ME 51143093, Vanillin, traceable to primary standards (LGC); Vanillin melting point standard, Pharmaceutical Secondary Standard; Certified Reference Material
|
|
| CAS | 121-33-5 | |
| PubChem CID | 1183 | |
| ChEMBL ID | CHEMBL13883 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 152.15 | ALogp: | 1.2 |
| HBD: | 1 | HBA: | 3 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 46.5 | Aromatic Rings: | 1 |
| Heavy Atoms: | 11 | QED Weighted: | 0.656 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.484 | MDCK Permeability: | 0.00001370 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.082 |
| Human Intestinal Absorption (HIA): | 0.012 | 20% Bioavailability (F20%): | 0.003 |
| 30% Bioavailability (F30%): | 0.216 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.745 | Plasma Protein Binding (PPB): | 79.00% |
| Volume Distribution (VD): | 0.805 | Fu: | 18.19% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.84 | CYP1A2-substrate: | 0.731 |
| CYP2C19-inhibitor: | 0.081 | CYP2C19-substrate: | 0.217 |
| CYP2C9-inhibitor: | 0.032 | CYP2C9-substrate: | 0.775 |
| CYP2D6-inhibitor: | 0.019 | CYP2D6-substrate: | 0.553 |
| CYP3A4-inhibitor: | 0.045 | CYP3A4-substrate: | 0.231 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.655 | Half-life (T1/2): | 0.885 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.035 | Human Hepatotoxicity (H-HT): | 0.019 |
| Drug-inuced Liver Injury (DILI): | 0.033 | AMES Toxicity: | 0.067 |
| Rat Oral Acute Toxicity: | 0.031 | Maximum Recommended Daily Dose: | 0.097 |
| Skin Sensitization: | 0.26 | Carcinogencity: | 0.119 |
| Eye Corrosion: | 0.976 | Eye Irritation: | 0.993 |
| Respiratory Toxicity: | 0.964 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000027 |  |
0.714 | D0E9CD | 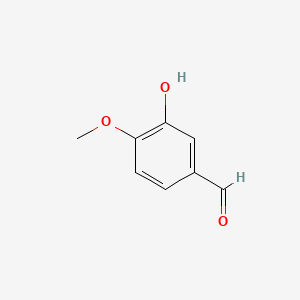 |
0.765 | ||
| ENC001101 |  |
0.619 | D0V9EN | 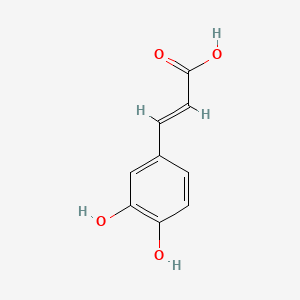 |
0.354 | ||
| ENC000172 |  |
0.583 | D03LGG | 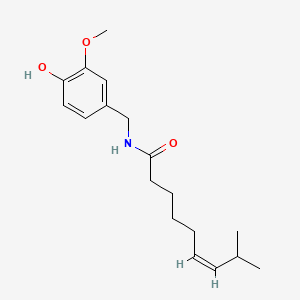 |
0.338 | ||
| ENC000296 |  |
0.550 | D0U5CE | 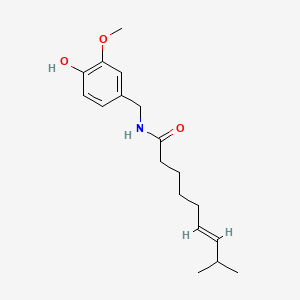 |
0.338 | ||
| ENC001056 |  |
0.550 | D0U0OT | 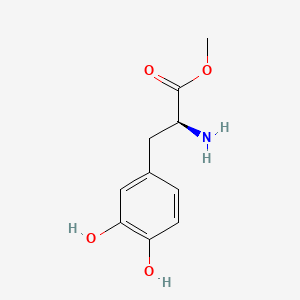 |
0.321 | ||
| ENC000095 |  |
0.537 | D0C4YC | 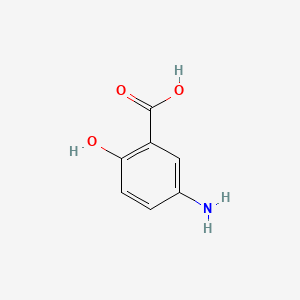 |
0.311 | ||
| ENC000777 |  |
0.512 | D09GYT | 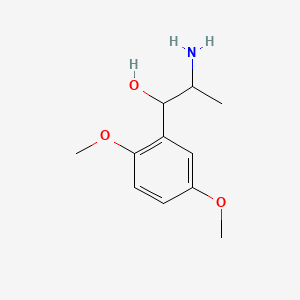 |
0.296 | ||
| ENC000507 |  |
0.500 | D06GIP |  |
0.283 | ||
| ENC004652 |  |
0.479 | D01WJL | 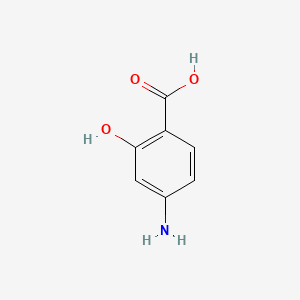 |
0.283 | ||
| ENC004988 |  |
0.478 | D0BA6T | 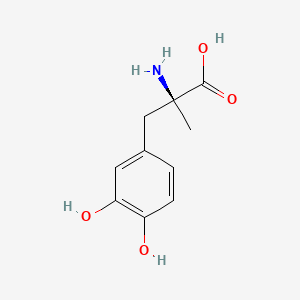 |
0.278 | ||