NPs Basic Information
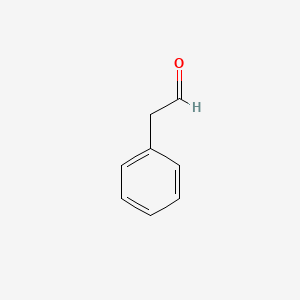
|
Name |
Phenylacetaldehyde
|
| Molecular Formula | C8H8O | |
| IUPAC Name* |
2-phenylacetaldehyde
|
|
| SMILES |
C1=CC=C(C=C1)CC=O
|
|
| InChI |
InChI=1S/C8H8O/c9-7-6-8-4-2-1-3-5-8/h1-5,7H,6H2
|
|
| InChIKey |
DTUQWGWMVIHBKE-UHFFFAOYSA-N
|
|
| Synonyms |
phenylacetaldehyde; 2-phenylacetaldehyde; 122-78-1; Benzeneacetaldehyde; Hyacinthin; Phenylethanal; alpha-Tolualdehyde; 2-Phenylethanal; Phenylacetic aldehyde; Oxophenylethane; alpha-Toluic aldehyde; Acetaldehyde, phenyl-; Benzylcarboxaldehyde; 1-Oxo-2-phenylethane; Phenacetaldehyde; phenyl acetaldehyde; phenyl-Acetaldehyde; .alpha.-Tolualdehyde; Benzenacetaldehyde; .alpha.-Toluic aldehyde; Phenylacetaldehyde (natural); alpha-Phenylacetaldehyde; FEMA No. 2974; Benzacetaldehyde; NSC 406309; UNII-U8J5PLW9MR; U8J5PLW9MR; alpha-Tolyaldehyde; EINECS 204-574-5; Acetaldehyde, phenyl- (8CI); a-Tolyaldehyde; CHEBI:16424; NSC-406309; DSSTox_CID_1483; DSSTox_RID_76177; DSSTox_GSID_21483; CAS-122-78-1; benzeneethanal; a-Tolualdehyde; AI3-02175; 2-phenylethanone; a-toluic aldehyde; Phenylacetoaldehyde; benzene acetaldehyde; a-Phenylacetaldehyde; 2-phenyl-acetaldehyde; bmse000427; NCIOpen2_003602; Phenylacetaldehyde, >=90%; SCHEMBL18972; PHENYLACETALDEHYDE [MI]; PHENYLACETALDEHYDE [FCC]; CHEMBL1233464; DTXSID3021483; FEMA NO. 2874; PHENYLACETALDEHYDE [FHFI]; ZINC895323; STR00412; Tox21_201582; Tox21_302945; MFCD00006993; NSC406309; s9357; AKOS000119316; CCG-266073; CS-W011205; DB02178; HY-W010489; Phenylacetaldehyde, >=95%, FCC, FG; NCGC00249076-01; NCGC00256522-01; NCGC00259131-01; DB-041686; FT-0631709; P0119; EN300-18996; C00601; D78329; 10.14272/DTUQWGWMVIHBKE-UHFFFAOYSA-N.1; A804962; Q424998; doi:10.14272/DTUQWGWMVIHBKE-UHFFFAOYSA-N.1; Q-201558; F2190-0653; Z104472146; D60A2590-0A65-4BA8-A05B-D8423408535C
|
|
| CAS | 122-78-1 | |
| PubChem CID | 998 | |
| ChEMBL ID | CHEMBL1233464 |
*Note: the IUPAC Name was collected from PubChem.
Chemical Classification: |
|
|
|---|
——————————————————————————————————————————
NPs Species Source
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference | |
|---|---|---|---|---|---|---|---|---|
| Endophyte ID | Endophyte Name | Family | Genus | Taxonomy ID | GenBank ID | Closest GenBank ID | Reference |
NPs Biological Activity
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bioactivity Name | Target ID | Target Name | Target Type | Target Organism | Target Organism ID | Potency of Bioactivity | Activity Type | Value | Unit | Endophyte ID | Endophyte Name |
NPs Physi-Chem Properties
| Molecular Weight: | 120.15 | ALogp: | 1.8 |
| HBD: | 0 | HBA: | 1 |
| Rotatable Bonds: | 2 | Lipinski's rule of five: | Accepted |
| Polar Surface Area: | 17.1 | Aromatic Rings: | 1 |
| Heavy Atoms: | 9 | QED Weighted: | 0.545 |
——————————————————————————————————————————
NPs ADMET Properties*
ADMET: Absorption
| Caco-2 Permeability: | -4.291 | MDCK Permeability: | 0.00003270 |
| Pgp-inhibitor: | 0 | Pgp-substrate: | 0.007 |
| Human Intestinal Absorption (HIA): | 0.005 | 20% Bioavailability (F20%): | 0.056 |
| 30% Bioavailability (F30%): | 0.002 |
——————————————————————————————————————————
ADMET: Distribution
| Blood-Brain-Barrier Penetration (BBB): | 0.933 | Plasma Protein Binding (PPB): | 36.94% |
| Volume Distribution (VD): | 1.575 | Fu: | 67.03% |
——————————————————————————————————————————
ADMET: Metabolism
| CYP1A2-inhibitor: | 0.699 | CYP1A2-substrate: | 0.448 |
| CYP2C19-inhibitor: | 0.225 | CYP2C19-substrate: | 0.3 |
| CYP2C9-inhibitor: | 0.024 | CYP2C9-substrate: | 0.115 |
| CYP2D6-inhibitor: | 0.157 | CYP2D6-substrate: | 0.479 |
| CYP3A4-inhibitor: | 0.018 | CYP3A4-substrate: | 0.251 |
——————————————————————————————————————————
ADMET: Excretion
| Clearance (CL): | 9.592 | Half-life (T1/2): | 0.784 |
——————————————————————————————————————————
ADMET: Toxicity
| hERG Blockers: | 0.097 | Human Hepatotoxicity (H-HT): | 0.059 |
| Drug-inuced Liver Injury (DILI): | 0.313 | AMES Toxicity: | 0.395 |
| Rat Oral Acute Toxicity: | 0.019 | Maximum Recommended Daily Dose: | 0.03 |
| Skin Sensitization: | 0.957 | Carcinogencity: | 0.319 |
| Eye Corrosion: | 0.973 | Eye Irritation: | 0.992 |
| Respiratory Toxicity: | 0.952 |
——————————————————————————————————————————
*Note: the ADMET properties was calculated by ADMETlab 2.0. Reference: PMID: 33893803.
Similar Compounds*
Compounds similar to EMNPD with top10 similarity:
| Similar NPs | Similar Drugs | ||||||
|---|---|---|---|---|---|---|---|
| NPs ID | NPs 2D Structure | Similarity Score | TTD ID | Drug 2D Structure | Similarity Score | ||
| ENC000014 |  |
0.581 | D05OIS |  |
0.581 | ||
| ENC000203 |  |
0.581 | D05BMG |  |
0.500 | ||
| ENC000205 |  |
0.581 | D0T3LF |  |
0.500 | ||
| ENC000219 |  |
0.543 | D0P9AC |  |
0.486 | ||
| ENC005854 |  |
0.543 | D0R1CR | 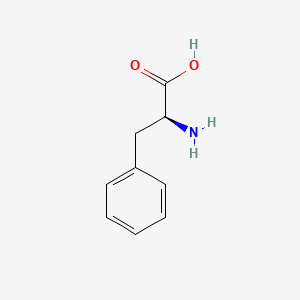 |
0.475 | ||
| ENC000218 |  |
0.543 | D0U0RZ |  |
0.474 | ||
| ENC000054 |  |
0.543 | D0P6UB |  |
0.462 | ||
| ENC000012 | 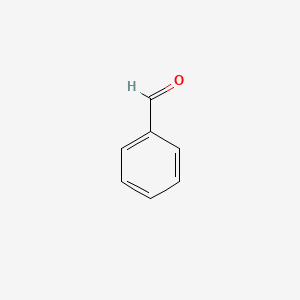 |
0.531 | D0P2GK |  |
0.452 | ||
| ENC000217 |  |
0.529 | D07ONP |  |
0.442 | ||
| ENC000128 |  |
0.529 | D0G1OZ | 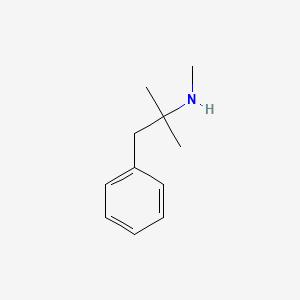 |
0.439 | ||