Drug General Information
| Drug Name |
Aripiprazole
|
||
| Synonyms |
129722-12-9; Abilify; Abilitat; Abilify Discmelt; OPC-14597; Discmelt; Opc 14597; OPC 31; OPC-31; 7-[4-[4-(2,3-Dichlorophenyl)-1-piperazinyl]butoxy]-3,4-dihydrocarbostyril; UNII-82VFR53I78; C23H27Cl2N3O2; HSDB 7320; 7-(4-(4-(2,3-DICHLOROPHENYL)PIPERAZIN-1-YL)BUTOXY)-3,4-DIHYDROQUINOLIN-2(1H)-ONE; CHEMBL1112; 7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}-1,2,3,4-tetrahydroquinolin-2-one; 7-(4-(4-(2,3-Dichlorophenyl)-1-piperazinyl)butyloxy)-3,4-dihydro-2(1H)-quinolinone; CHEBI:31236; Abilify; Aripiprazol; Aripiprazolum; Aripirazole; Arpizol; Asprito; Pripiprazole; Aripiprazole [USAN]; Otsuka brand of aripiprazole; OPC 14597; ALKS-9070; Abilify (TN); BMS-337039; Bristol-Myers Squibb brand of aripiprazole; Discmelt (TN); KS-1030; S06-0010; Aripiprazole (JAN/USAN/INN); 7-(4-(4-(2,3-Dichlorophenyl)-1-piperazinyl)butoxy)-3,4-dihydro-2(1H)-quinolinone; 7-(4-(4-(2,3-Dichlorophenyl)-1-piperazinyl)butoxy)-3,4-dihydrocarbostyril; 7-[4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydro-1H-quinolin-2-one; 7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}-3,4-dihydroquinolin-2(1H)-one; ALKS9070/ALKS9072; Aripiprazole/escitalopram fixed-dose combination
|
||
| Drug Type |
Small molecular drug
|
||
| Indications |
Erythropoietic porphyrias [ICD-11: 5C58.12]
|
Approved
|
|
|
Schizophrenia [ICD-11: 6A20]
|
Approved
|
||
|
Major depressive disorder [ICD-11: 6A70.3]
|
Phase 3
|
||
|
Bipolar disorder [ICD-11: 6A60]
|
Application submitted
|
||
| Company |
Bristol-Myers Squibb; Otsuka Pharmaceutical Co., Ltd
|
||
| Summary |
Aripiprazole is an atypical antipsychotic used in the treatment of a wide variety of mood and psychotic disorders, such as schizophrenia, bipolar I, major depressive disorder, irritability associated with autism, and Tourette's syndrome.
|
||
| Target |
Dopamine D2 receptor (D2R)
Mechanism of Action: Agonist
|
T67162 | |
Drug Chemical Infomation and External Links
|
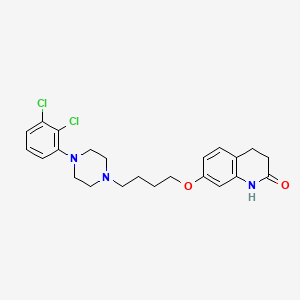
Formula |
C23H27Cl2N3O2
|
| Canonical SMILES |
C1CC(=O)NC2=C1C=CC(=C2)OCCCCN3CCN(CC3)C4=C(C(=CC=C4)Cl)Cl
|
|
| InChI |
1S/C23H27Cl2N3O2/c24-19-4-3-5-21(23(19)25)28-13-11-27(12-14-28)10-1-2-15-30-18-8-6-17-7-9-22(29)26-20(17)16-18/h3-6,8,16H,1-2,7,9-15H2,(H,26,29)
|
|
| InChIKey |
CEUORZQYGODEFX-UHFFFAOYSA-N
|
|
| CAS Number | CAS 129722-12-9 | |
| TTD ID | D0H3HM | |
| DrugBank ID | DB01238 | |
| PubChem Compound ID | 60795 | |
| PubChem Substance ID |
582954
,
7848227
,
7978512
,
8187059
,
11528738
,
12014488
,
14759758
,
15103350
,
25819894
,
26719891
,
29215485
,
29215486
,
43118141
,
46386763
,
46505745
,
49658671
,
49666283
,
49666418
,
49681659
,
49830718
,
57314118
,
77392862
,
81042514
,
81065479
,
81092781
,
85202210
,
85209988
,
89736075
,
92307952
,
92308408
,
92308936
,
92713933
,
103307946
,
104021727
,
104321668
,
117470150
,
117868935
,
118048569
,
124365869
,
124658892
,
124799564
,
125001911
,
125339803
,
126525334
,
126592371
,
126624438
,
126655782
,
126669992
,
126728368
,
127275956
|
|
| ChEBI ID | CHEBI:31236 | |
| ADReCS Drug ID | BADD_D00165 |
Drug-Food (Herb) Interactions
- Drug
- Foods
- Herbs
- Positive
- Negative
- Possible
- Harmful
- No Effect
| ID | Name | Dose | Type | Drug Brand Name | Drug Dose | Drug Dosage Form | Experimental Species | Individuals Number | Test Sample | Ingredient | Effect | Relationship Classification | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Name | Dose | Type | Drug Brand Name | Drug Dose | Drug Dosage Form | Experimental Species | Individuals Number | Test Sample | Ingredient | Effect | Relationship classification |