Drug General Information
| Drug Name |
Quinapril
|
||
| Synonyms |
Ectren; Koretic; Quinaprilum; QUINAPRIL HCL; Quinaprilum [Latin]; Accupril (TN); Quinapril [INN:BAN]; Quinapril (USP/INN); [3S-[2[R*(R*)],3R*]]-2-[2-[[1-(ethoxycarbonyl)-3-phenylpropyl]amino]-1-oxopropyl]-1,2,3,4-tetrahydro-3-Isoquinolinecarboxylic acid; (3S)-2-(N-{(1S)-1-[(ethyloxy)carbonyl]-3-phenylpropyl}-L-alanyl)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid; (3S)-2-[(2S)-2-[[(2S)-1-ethoxy-1-oxo-4-phenylbutan-2-yl]amino]propanoyl]-3,4-dihydro-1H-isoquinoline-3-carboxylic acid; (3S)-2-{(2S)-2-[(1S)-1-ethoxycarbonyl-3-phenylpropylamino]propanoyl}-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid; (3S)-2-{N-((S)-1-Ethoxycarbonyl-3-phenylpropyl)-L-alanyl}-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid
|
||
| Drug Type |
Small molecular drug
|
||
| Indications |
Hypertension [ICD-11: BA00-BA04]
|
Approved
|
|
| Company |
Pfizer Pharmaceuticals
|
||
| Summary |
Quinapril is an ACE inhibitor prodrug used to treat hypertension, congestive heart failure, and slow rate of progression of renal disease.
|
||
| Target |
Angiotensin-converting enzyme (ACE)
Mechanism of Action: Inhibitor
|
T82577 | |
Drug Chemical Infomation and External Links
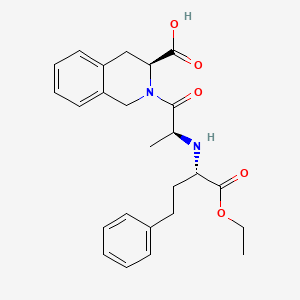
|
Formula |
C25H30N2O5
|
| Canonical SMILES |
CCOC(=O)C(CCC1=CC=CC=C1)NC(C)C(=O)N2CC3=CC=CC=C3CC2C(=O)O
|
|
| InChI |
1S/C25H30N2O5/c1-3-32-25(31)21(14-13-18-9-5-4-6-10-18)26-17(2)23(28)27-16-20-12-8-7-11-19(20)15-22(27)24(29)30/h4-12,17,21-22,26H,3,13-16H2,1-2H3,(H,29,30)/t17-,21-,22-/m0/s1
|
|
| InChIKey |
JSDRRTOADPPCHY-HSQYWUDLSA-N
|
|
| CAS Number | CAS 85441-61-8 | |
| TTD ID | D0I7SZ | |
| DrugBank ID | DB00881 | |
| PubChem Compound ID | 54892 | |
| PubChem Substance ID |
9602
,
7980446
,
8145927
,
8149893
,
8183841
,
11335885
,
11361124
,
11364491
,
11367053
,
11369615
,
11372621
,
11374133
,
11377777
,
11462096
,
11484485
,
11488641
,
11491366
,
11492542
,
11495411
,
14759220
,
14881354
,
17397841
,
34718828
,
46506309
,
47589015
,
48035134
,
48259244
,
48416506
,
49835285
,
50065313
,
50113005
,
50113006
,
50744269
,
85787397
,
92309221
,
93166955
,
99301798
,
103590286
,
104306102
,
117576967
,
121264493
,
124893433
,
126683866
,
127334979
,
127334980
,
127334981
,
127334982
,
127334983
,
127334984
,
127334985
|
|
| ChEBI ID | CHEBI:8713 | |
| ADReCS Drug ID | BADD_D01890 |
Drug-Food (Herb) Interactions
- Drug
- Foods
- Herbs
- Positive
- Negative
- Possible
- Harmful
- No Effect
| ID | Name | Dose | Type | Drug Brand Name | Drug Dose | Drug Dosage Form | Experimental Species | Individuals Number | Test Sample | Ingredient | Effect | Relationship Classification | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Name | Dose | Type | Drug Brand Name | Drug Dose | Drug Dosage Form | Experimental Species | Individuals Number | Test Sample | Ingredient | Effect | Relationship classification |