Drug General Information
| Drug Name |
Gliclazide
|
||
| Synonyms |
Diaikron; Diamicron; Gliclazida; Gliclazidum; Gliklazid; Glimicron; Glyclazide; Nordialex; S 1702; S 852; SE 1702; Diamicron (TN); Dianorm (TN); Gliclazida [INN-Spanish]; Gliclazidum [INN-Latin]; Glimicron (TN); S-1702; S-852; Gliclazide (JAN/INN); Glubitor-OD (TN); Gliclazide [BAN:INN:DCF:JAN]; N-(hexahydrocyclopenta[c]pyrrol-2(1H)-ylcarbamoyl)-4-methylbenzenesulfonamide; N-[(hexahydrocyclopenta[c]pyrrol-2(1H)-ylamino)carbonyl]-4-methylbenzenesulfonamide; N-(4-Methylbenzenesulfonyl)-N'-(3-azabicyclo(3.3.0)oct-3-yl)urea; 1-(3,3a,4,5,6,6a-hexahydro-1H-cyclopenta[c]pyrrol-2-yl)-3-(4-methylphenyl)sulfonylurea; 1-(3-Azabicyclo(3.3.0)oct-3-yl)-3-(p-tolylsulfonyl)urea; 1-(3-Azabicyclo[3.3.0]oct-3-yl)-3-p-tolylsulphonylurea; 1-(Hexahydrocyclopenta(c)pyrrol-2(1H)-yl)-3-(p-tolylsulfonyl)urea
|
||
| Drug Type |
Small molecular drug
|
||
| Indications |
Diabetic complication [ICD-11: 5A2Y]
|
Approved
|
|
| Company |
NA
|
||
| Summary |
Gliclazide is a sulfonylurea used to treat hyperglycemia in patients with type 2 diabetes mellitus.
|
||
| Target |
ATP-binding cassette transporter C8 (ABCC8)
Mechanism of Action: Blocker
|
T91480 | |
Drug Chemical Infomation and External Links
|
Formula |
C15H21N3O3S
|
| Canonical SMILES |
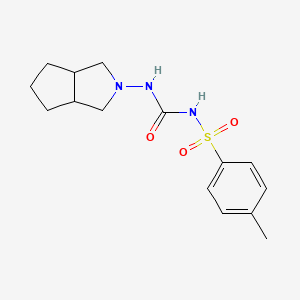
CC1=CC=C(C=C1)S(=O)(=O)NC(=O)NN2CC3CCCC3C2
|
|
| InChI |
1S/C15H21N3O3S/c1-11-5-7-14(8-6-11)22(20,21)17-15(19)16-18-9-12-3-2-4-13(12)10-18/h5-8,12-13H,2-4,9-10H2,1H3,(H2,16,17,19)
|
|
| InChIKey |
BOVGTQGAOIONJV-UHFFFAOYSA-N
|
|
| CAS Number | CAS 21187-98-4 | |
| TTD ID | D0M2MR | |
| DrugBank ID | DB01120 | |
| PubChem Compound ID | 3475 | |
| PubChem Substance ID |
3206372
,
5035155
,
7848662
,
7979407
,
8152209
,
10321809
,
11342141
,
11362324
,
11364372
,
11366934
,
11369496
,
11372903
,
11374235
,
11377658
,
11466586
,
11467706
,
11486292
,
11487726
,
11491677
,
11492413
,
11495292
,
11533945
,
14826273
,
24895090
,
26612635
,
26612723
,
26748918
,
26748919
,
26748920
,
29222608
,
47656599
,
47730748
,
48179242
,
48179243
,
48328563
,
48403959
,
48416054
,
49646130
,
49698833
,
50061218
,
50086570
,
50107481
,
50107482
,
50107483
,
50880518
,
57321824
,
81040947
,
81066070
,
81093129
,
85280584
|
|
| ChEBI ID | CHEBI:31654 | |
| ADReCS Drug ID | BADD_D01022 |
Drug-Food (Herb) Interactions
- Drug
- Foods
- Herbs
- Positive
- Negative
- Possible
- Harmful
- No Effect
| ID | Name | Dose | Type | Drug Brand Name | Drug Dose | Drug Dosage Form | Experimental Species | Individuals Number | Test Sample | Ingredient | Effect | Relationship Classification | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Name | Dose | Type | Drug Brand Name | Drug Dose | Drug Dosage Form | Experimental Species | Individuals Number | Test Sample | Ingredient | Effect | Relationship classification |